Black body radiation refers to the electromagnetic radiation emitted by a perfect black body, which absorbs all incident radiation and re-emits it as thermal radiation, with a spectrum that depends solely on its temperature.

Electromagnetic radiation falling on an object (body) at a particular temperature can be absorbed, reflected or transmitted by the body. At the same time, a body also emits electromagnetic radiation as part of a conversion of the body’s internal energy to electromagnetic energy.
In fact, a body at thermal equilibrium with its surroundings emits the same amount of radiation of a particular frequency as it absorbs. Otherwise, a body that absorbs radiation better than it emits, or vice versa, will not be in thermal equilibrium with its surroundings. This is known as Kirchhoff’s law of thermal radiation. As the amount of radiation at a particular frequency emitted by a body at a particular temperature varies between different objects, Kirchhoff suggested a reference body to study how matter emits electromagnetic radiation. He hypothesised an ideal body called a black body, which at thermal equilibrium absorbs and emits all frequencies of radiation.
Since then, scientists attempted to design practical models that approximates a black body. Most designs consist of a cavity with a pinhole so small that any electromagnetic radiation that goes into the cavity has very little chance of getting out. Therefore, such a device is considered an ideal absorber of all frequencies of radiation, i.e. one that absorbs more energy via the pinhole than any other body at the same temperature.
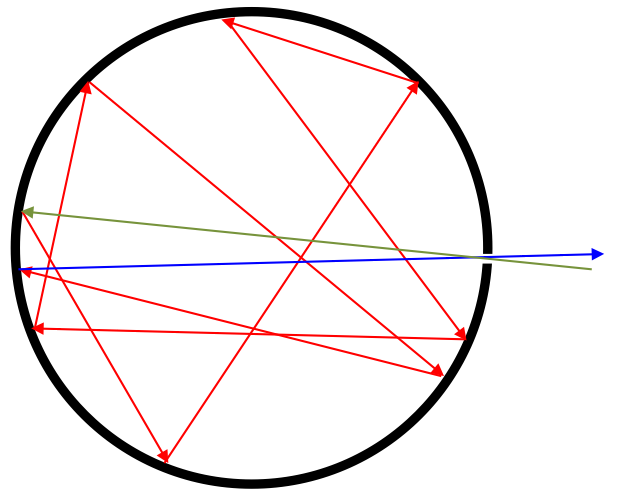
In the diagram above, the green arrow represents radiation that enters the cavity though the hole. It is absorbed by the wall of the cavity and re-emitted into the cavity. The re-emitted radiation then gets absorbed and re-emitted so many times within the cavity (red lines) that it attains thermal equilibrium with the cavity wall. Radiation that finally escapes the cavity through the hole (blue line) is passed through a prism to separate the various frequencies, which are measured and analysed. Since the device is deemed an ideal absorber of all frequencies of radiation, it must, according to Kirchhoff’s law of thermal radiation, be an ideal emitter of all frequencies of radiation too.
Enhancements were regularly made to the designs. In 1898, about forty years after Kirchhoff’s suggestion, Otter Lummer and Ferdinand Kurlbaum, both German physicists, constructing a device that significantly improved the measurement of black body radiation. The emitted radiation had the following frequency distribution at various temperatures:
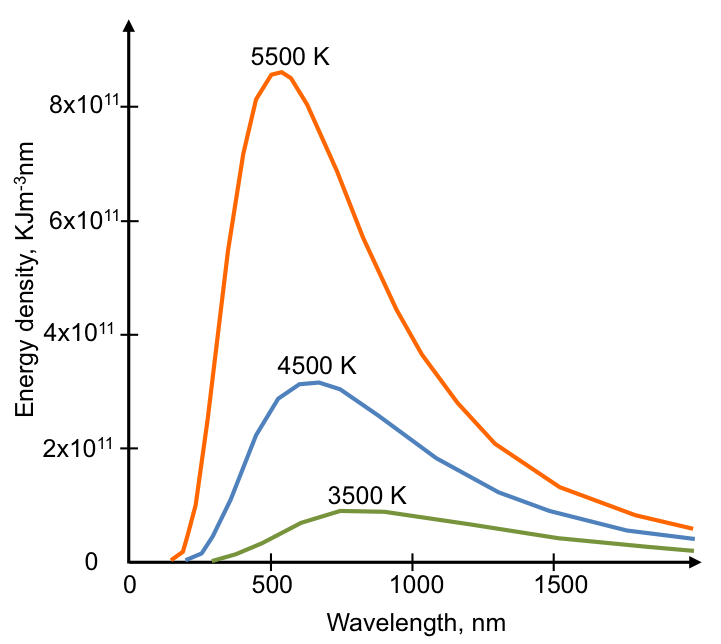
The subsequent challenge was to express the distribution profiles mathematically (see Planck’s law).

Question
With reference to the graph above, cite an example to explain the shift of the peak of an energy-frequency distribution curve to shorter wavelengths at higher temperatures.
Answer
An iron rod glows red when it is heated. This is due to the iron absorbing and re-emitting radiation with wavelength predominantly at the 680-700 nm range.

As it is heated further, it turns white as the iron now absorbs and re-emits radiation over a broader range of wavelengths from 470nm (blue) to 700 nm (red), which combine to form white light. At an even higher temperature, the rod emits blue light because the peak of the distribution curve has narrowed and shifted to the blue wavelength of 470 nm.
