The Born interpretation states that the probability of finding an electron of a certain quantum state around a point is proportional to the square of the modulus of the electron’s wave function , which is called the probability density. It was proposed by Max Born, a German physicist, in 1926.

The amplitude of a wave function represents its magnitude, e.g. is the amplitude in the function
. Since the quantum state of an electron is completely described by its wave function
, which depends on the position
of the electron , Born drew a parallel between the probability of finding a particle in a region of space and the intensity of a classical wave, which is proportional to the square of the wave’s amplitude.

Question
Using Hooke’s law, F = –kx, show that the intensity of a wave is proportional to the square of its amplitude.
Answer
Substituting Hooke’s law in dE = –Fdx, we have dE = kxdx (where E is energy). Integrating the simple harmonic motion over a maximum amplitude A,
Since intensity I is defined as , we have
for an electromagnetic radiation falling on a particular area of a material over a certain duration.
Mathematically, the Born interpretation is:
or to ensure that the sum of individual probabilities of locating an electron over all space is normalised to one. The wave function of an electron is a complex quantity and therefore the above equation can be written as:
where is the complex conjugate of
.

Question
Why is the wave function a complex quantity? Show that .
Answer
Some differential equations are easier to solve when the function is in the complex form. This happens to be the case for the Schrodinger equation. However, only the real component is used when values of the function are compared with experimental data.
Let . So
.
Therefore, .
In the previous article, we mentioned that Schrodinger did not have a convincing physical interpretation of the wave function ψ, other than it being a wave equation of an electron in the atom. With the Born interpretation, the electron probability density function for a unique combination of the three quantum numbers
,
and
, mathematically describes an atomic orbital. When we plot the probability functions using a mathematical software, we obtain the following shapes:
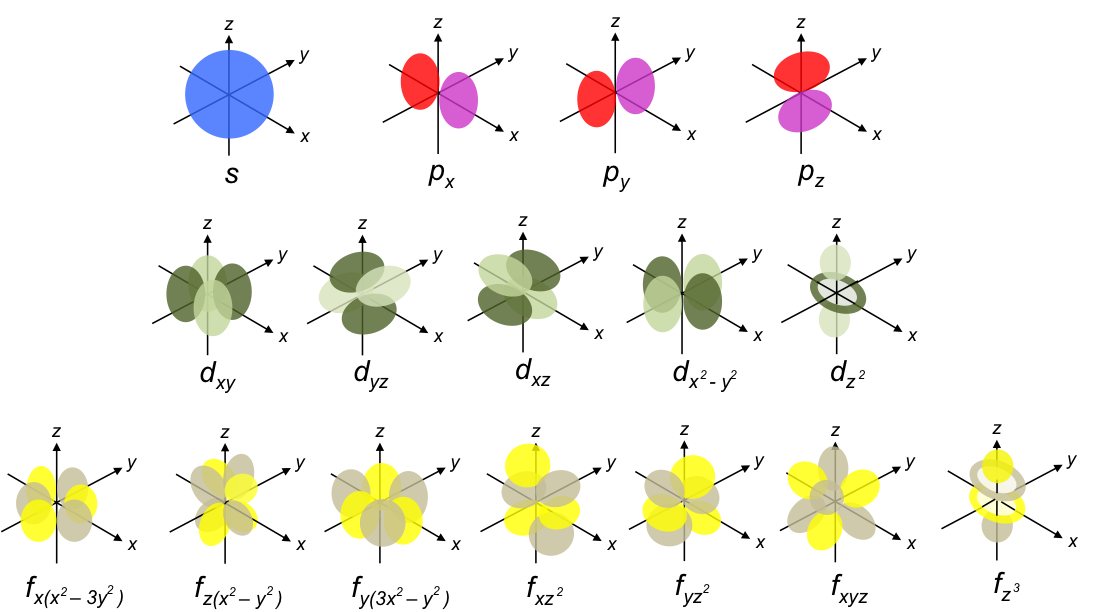
These are the orbitals of a hydrogen atom.

