What are the principles of separation in paper chromatography?
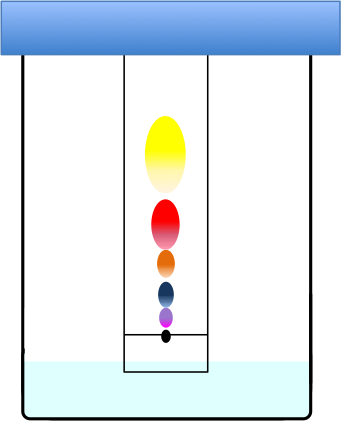
The distances travelled by the chemical components of a mixture in paper chromatography are affected by propelling and retardation forces.
|
Propelling force |
Solvent-flow along the length of the paper by capillary action |
|
Retardation forces |
Partitioning of chemical components between the mobile phase and the stationary phase |
|
Adsorption of chemical components onto cellulose |
|
|
Ion-exchange bonding of chemical components with cellulose |
Filter paper consists of almost pure cellulose, which is a polymer of D-glucose. However, some functional groups on the polymeric chain may be oxidised to the carboxyl form as shown in the diagram below.
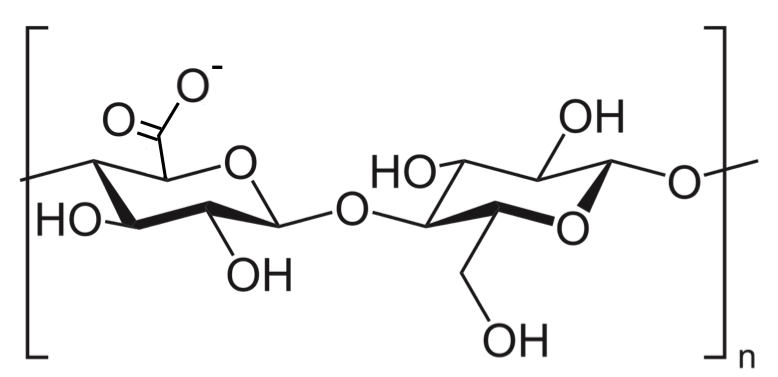
The hydroxyl, carboxyl and ether groups on cellulose interact with water molecules in the atmosphere via hydrogen bonds and van der Waals forces to form a multilayer of water called the stationary phase.
The solvent, known as the mobile phase, is usually selected to be relatively less polar than water so that the components to be separated dissolve in different amounts in each phase. As the chemical components are propelled by the solvent along the paper via capillary action, they are partitioned between the stationary and mobile phases; that is, the chemical components equilibrate and distribute themselves between the two phases according to their solubilities in each phase.

Question
What is capillary action?
Answer
Capillary action is the spontaneous movement of a liquid through narrow spaces or porous materials, often against gravity. It occurs when the attraction between the liquid and a solid surface is stronger than the internal forces holding the liquid together. Although the solid cellulose surface may already have a thin layer of adsorbed water molecules, the structure remains porous, with the interconnected pores resembling small capillary tubes that allow additional solvent to move through it.
Solvent molecules near the liquid–solid interface interact with cellulose molecules not only beside them at the same height but also higher up along the surrounding surface. These adhesive interactions cause the solvent to wet the cellulose surface and climb slightly along it, producing an upward-curving surface (a concave meniscus).
Every molecule on that curved surface is being pulled by its neighbours along the line of the curve. Because the surface is concave, the net result of all these sideways pulls is a resultant force directed upwards. This lifts the solvent column upwards until it is balanced by gravity.
The moving components are slowed down by the partitioning process because they are held back by stationary polar water molecules (via van der Waals forces and hydrogen bonds) when they dissolve and equilibrate in the stationary phase. Eventually, the partition process may bring the components to a stop over a certain length of the paper.
The more soluble a component is in the mobile phase (relatively less polar) versus the stationary phase (relatively more polar), the further it is propelled along the paper by the mobile phase. Furthermore, direct adsorption on to the cellulose functional groups via hydrogen bonding and ion-exchange bonding with the carboxyl and possibly hydroxyl groups also serve to slow down and separate the chemical components according to their affinity with these functional groups.