The pH range of an indicator is the pH interval in which the indicator changes colour.
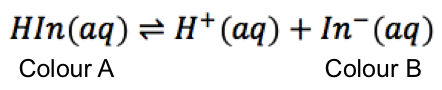
To elaborate further, we will use the Henderson-Hasselbalch equation to study the equilibrium of a pH indicator, which is a weak acid.
Consider a titration using two drops of bromocresol green with pKin = 4.7. We have
Since the concentration of the indicator in the analyte is very low, the contribution of H+ from the indicator is negligible and does not affect the total concentration of H+ in the solution. Hence, the pH value in eq1 is solely due to the H+ of the analyte; that is, the pH of the analyte determines the position of the equilibrium of the indicator.
When bromocresol green is predominantly in the form HIn, it appears yellow. If it is predominantly in the form In–, we see it as blue.
As a rule of thumb, our eyes can perceive a complete change in the colour of the indicator from its acid form to the conjugate base and vice versa, when the concentration of one form is ten times greater than the other. So, bromocresol green appears blue when:
which corresponds to the indicator in a pH environment of pH ≥ 5.7 (by substituting eq2 in eq1).
At the other limit, bromocresol green appears yellow when:
which corresponds to the indicator in a pH environment of pH ≤ 3.7 (by substituting eq3 in eq1).
In other words, if a few drops of bromocresol green are added to an analyte with a pH ≥ 5.7 and another with a pH ≤ 3.7, the solutions will appear blue and yellow, respectively.
We call this difference in pH (3.7 to 5.7 for bromocresol green) the pH range of the indicator.