Saturated vapour pressure is the pressure exerted by a vapour in equilibrium with its liquid or solid phase at a given temperature in a closed system. It represents the point at which the rate of particles leaving the condensed phase to enter the vapour phase equals the rate at which vapour particles condense back. This balance depends critically on temperature, as the energy available to the particles governs their ability to escape into the gas phase.

In a liquid (or solid), the particles have a range of kinetic energies, with their average energy determined by the temperature. As temperature increases, so does this average energy. However, even at a fixed temperature, some particles possess more energy than others. Among these, the more energetic particles near the surface can overcome the intermolecular forces holding them in the liquid (or solid) and escape into the vapour phase (see diagram above). As more particles accumulate in the vapour, some will lose energy through collisions and return to the liquid (or solid). The vapour particles move freely and, when they strike the walls of the container, they exert a pressure. Eventually, the rates of evaporation and condensation become equal, establishing a dynamic equilibrium. At this point, the pressure exerted by the vapour remains constant and is known as the saturated vapour pressure.
Measuring saturated vapour pressure
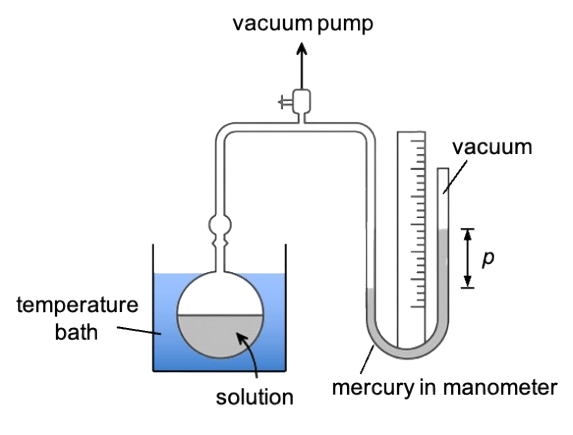
One of the most common techniques for measuring the saturated vapour pressure of a liquid or solid is the manometric method (see diagram below). In a typical setup, a known amount of the substance (liquid or solid) is placed in a sealed, evacuated chamber that is connected to a manometer. The system is kept at a constant and controlled temperature using a thermostatic bath, ensuring that any pressure changes are solely due to the substance’s vapour pressure at that temperature. As the substance begins to evaporate or sublimate, its vapour fills the chamber. The manometer, often a U-tube containing mercury or another fluid, registers the pressure exerted by the vapour as it reaches equilibrium with its condensed phase.
As more molecules escape from the surface of the liquid (or solid), the pressure inside the chamber increases. Eventually, the rate of molecules entering the vapour phase equals the rate returning to the liquid or solid—the point of dynamic equilibrium. At this stage, the manometer reading stabilises, indicating that the saturated vapour pressure has been reached.
Applications
The boiling point of a liquid is the temperature at which its saturated vapour pressure equals the external atmospheric pressure. At this temperature, bubbles of vapour can form within the bulk of the liquid, leading to boiling. This relationship explains why liquids boil at lower temperatures at higher altitudes — where atmospheric pressure is reduced — and why pressure cookers, which increase external pressure, raise the boiling point and cook food faster. Mathematically, the Clausius-Clapeyron equation describes the relationship between vapour pressure and temperature.

Question
Why do food cook faster when the boiling point of water is raised in a pressure cooker?
Answer
Inside a pressure cooker operating at increased pressure, the temperature of the steam and liquid water rises above 100°C — typically around 120°C at about 2 atmosphere. This means that the food inside the pressure cooker is essentially cooking in an environment at 120°C instead of 100°C.
Sublimation is the process where a solid transitions directly to a vapour without passing through the liquid phase. This occurs when the saturated vapour pressure of the solid equals or exceeds the surrounding atmospheric pressure at a given temperature. Just like with liquids, solids possess a saturated vapour pressure that increases with temperature. Substances like dry ice (solid carbon dioxide) and iodine readily sublimate at atmospheric pressure due to their relatively high vapour pressures even in the solid state. The point at which the solid and vapour are in equilibrium is also described by the Clausius-Clapeyron relation .