A pH indicator is usually a weak organic acid that changes colour according to the pH of the solution it is added to. As such, it serves as a useful marker of the pH of a solution, especially during the course of a titration.
Being a weak acid, a pH indicator HIn partially dissociates in a solution to give H+ and its conjugate base In–, with the undissociated acid and its conjugate base having different colours.
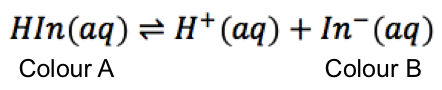
In a titration experiment, a few drops of a pH indicator, e.g. methyl orange (see below diagram), is added to the solution in the conical flask. If the solution is an acid, methyl orange, according to Le Chatelier’s principle, will be predominantly in the undissociated form, resulting in the solution being red.
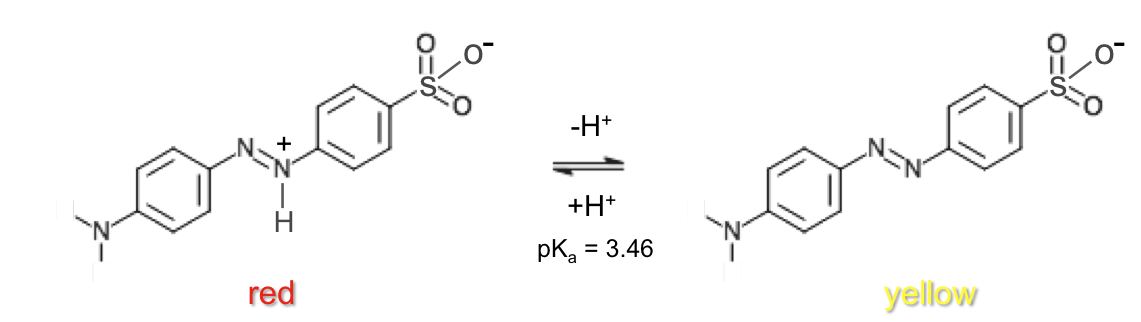
As a base is gradually added to the solution, the above equilibrium shifts to the right. With more conjugate base formed, the mixture of HIn and In– makes the solution orange, which for a strong acid-strong base titration indicates that the end point is very near. Thereafter, a couple of drops of base turn the solution yellow and complete the titration.
The following table lists some common indicators that are suitable for various acid-base titrations:
|
Burette |
Conical
flask |
Indicator | Colour change |
|
Strong acid |
Strong base |
Methyl orange |
Yellow → Red |
|
Strong base |
Strong acid |
Red → Yellow |
|
|
Strong acid |
Weak base |
Methyl red |
Yellow → Red |
|
Weak base |
Strong acid |
Red → Yellow |
|
|
Strong base |
Weak acid |
Phenolphthalein |
Colorless → Purple-pink |
| Weak acid | Strong base |
Purple-pink → Colorless |
If you are interested to find out more about how to choose an appropriate pH indicator, you can read this article and its related articles in the intermediate section.
