As mentioned in the section on collision theory, the energies of a gaseous reactant are distributed over a range that can be described by the Maxwell-Boltzmann distribution. These energies are mainly kinetic energies, which means that the gas particles are moving with different velocities. When we increase the temperature of a reaction mixture, we increase the average kinetic energy of the particles and hence their average velocities. This results in a higher rate of effective collisions between the reactants and therefore a higher rate of reaction.
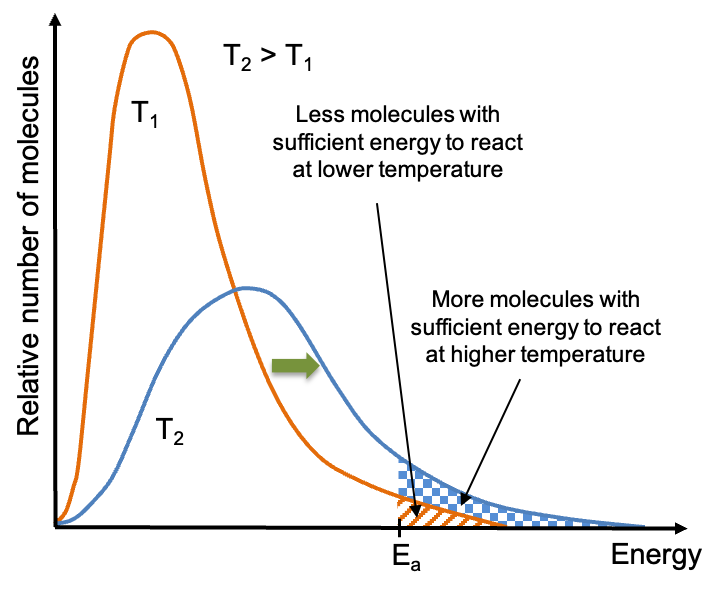
The energy profiles of a reactant at various temperatures are shown on the Maxwell-Boltzmann distribution diagram above. At higher temperatures, the energy distribution curve shifts to the right with more reactant molecules reaching and exceeding the activation energy, Ea, of the reaction. Since the total number of molecules for the two curves must be the same (i.e. the area under the curve at T1 must be equal to that at T2), the curve at the higher temperature, with respect to the one at the lower temperature, is expected to flatten as the number of molecules with higher energies increases.
The effect of temperature on the rate of reactions can be observed in everyday life, for example, the spoiling of a bottle of milk that is left in the summer sun and the preservation of animal corpses that are buried deep in snow.