An ionic equation is a simplified version of a chemical equation that involves ions.
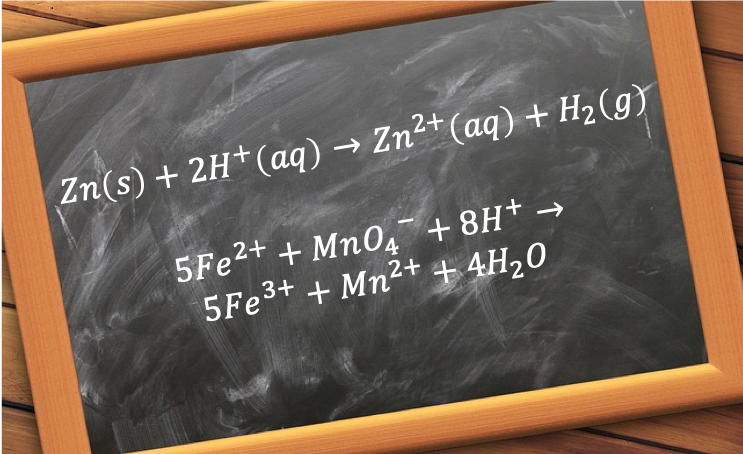
The construction of an ionic equation is usually accomplished in three steps, which can be illustrated using the example of the reaction between solid zinc and a solution of sulphuric acid:
Step 1: Write the full equation
Step 2: Rewrite the equation such that the aqueous species are in their dissociated forms
Step 3: Remove ions that appear on both sides of the equation (such ions are known as spectator ions)
The purpose of writing an ionic equation is to highlight the chemical species that are playing an active role in a reaction, which for the above reaction are Zn(s) and H+(aq) for the products, and Zn2+(aq) and H2(g) for the reactants, with SO42-(aq) playing the ‘inactive’ or spectator role.