The concentration of a chemical species in a solution can be expressed in a few ways, namely,
i) Molar concentration or molarity
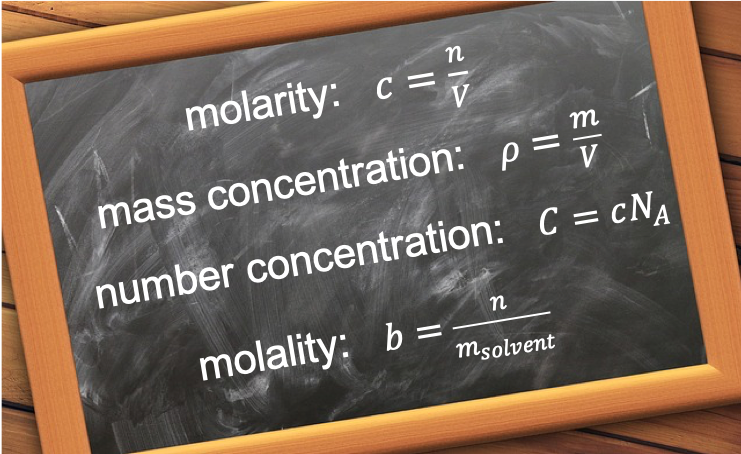
The molar concentration, c, of a solute (i.e. a chemical species in a solvent) is defined as
where n is the number of moles of the solute and V is the volume of the solvent. V is usually expressed in units of L, dm3 or cm3, which means that the molar concentration, c, has units of mol L-1, mol dm-3 or mol cm-3, respectively, even though the SI unit for c is mol m-3. As the units mol L-1 and mol dm-3 are equivalent and most commonly used, they are given the symbol M, which is called molar (not to be confused with molar mass, M). For example,
Q: How many moles of NaCl are there in 250.0 mL of a 0.100 M sodium chloride solution?
A:
ii) Mass concentration
The mass concentration, ρ, of a solute is defined as
where m is the mass of the solute in grams and V is the volume of the solvent, usually in dm3 or cm3. For a pure chemical, e.g. water or molten iron, the mass concentration equals its density. A chemical species’ mass concentration can be converted to its molarity by dividing it with its molar mass, M:
iii) Number concentration
The number concentration (or number density), C, of a solute is defined as
where NA is the Avogadro constant. Number concentration is used when the number of particles in a particular volume is countable, e.g. macromolecules.
iv) Molality
The molality, b, of a solute is defined as
where msolvent is the mass of the solvent and the units for b is mol kg-1. Molality is sometimes preferred over molarity in measuring the concentration of a chemical species, as the latter varies according to temperature due to thermal expansion.