The root mean square speed of a gas provides a measure of the average molecular speed in the gas.
Consider a gas particle of mass moving in a container to the right with velocity
(see diagram below). The change in momentum of the particle
in the
-coordinate before and after colliding elastically with a surface of the container is
with the particle colliding the same surface every
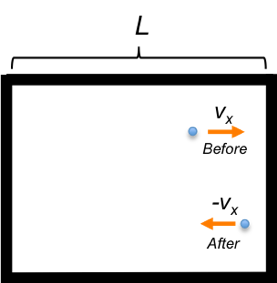
The force acting on the particle at collision is
By Newton’s third law, the force acting on the surface of the container by the particle is
The total force exerted on the surface by
particles is
where .
Comparing and the definition of root mean square speed, we have
, i.e. the square of the root mean square speed of the gas in the
-direction.
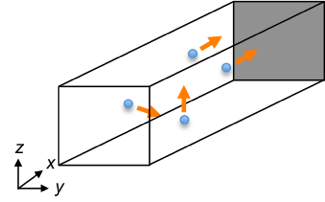
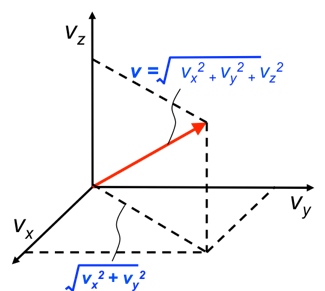
If the container is three-dimensional (see above diagram), the velocity vector for a particle in Cartesian coordinates in three dimensions is:
Even though a particle has three velocity components in space, the force exerted on the shaded wall is dependent only on the -component velocity of the particle.
To express the root mean square speed for particles, let’s begin by summing the squares of the velocity vectors of all particles:
Next, group the velocity components and divide the equation throughout by ,
The velocity components of the particles in the container are assumed to be random, with the particles evenly distributed in the container at any time. , like
and
, can be positive or negative, but their squares,
,
and
, are always positive. If the velocity components of the particles in the container are random, and the number of particles is very large,
Otherwise, there is a preference of direction in a particular axis and the particles’ movement is no longer random (you can convince yourself the validity of eq8 by summing the squares of three sets of randomly generated numbers in a spreadsheet and finding their averages; the larger the number of elements in each set, the closer the average values are to each other). Hence, we can rewrite eq7 as:
Substituting eq9 in eq5 gives
The pressure (not to be confused with momentum) exerted by
particles on the shaded wall is
where is the volume of the container.
Hence,
Substituting the ideal gas law in eq11 yields
Since (see this article for derivation ) and
,
Since and the root mean square speed is
, we have
. Therefore,
where is the molar mass of the gas.
