Spin-orbit coupling is the interaction between a particle’s spin angular momentum and orbital angular momentum. An electron orbiting around the nucleus ‘sees’ the nucleus circling it, just as a person on earth perceives the sun circling the earth while the latter orbits around the sun.

This apparent nuclear orbit creates a magnetic field  that exerts a torque on the electron’s spin magnetic dipole moment
that exerts a torque on the electron’s spin magnetic dipole moment  , resulting in an additional term of
, resulting in an additional term of  (where
(where  ) in the multi-electron Hamiltonian. To derive this term, we consider a 1-electron atom.
) in the multi-electron Hamiltonian. To derive this term, we consider a 1-electron atom.
Let  be the orbital angular momentum of the electron and
be the orbital angular momentum of the electron and  be the proton’s current loop, which generates a magnetic field of magnitude
be the proton’s current loop, which generates a magnetic field of magnitude  given by the Biot-Savart law. Since
given by the Biot-Savart law. Since  , we have
, we have

Substitute eq259 in  , we have,
, we have,  or
or  , where
, where  and
and  are unit vectors. Since
are unit vectors. Since  and
and  point in the same direction,
point in the same direction,  . Multiplying both sides of
. Multiplying both sides of  by
by  , we have
, we have  , which we substitute in eq65 (where
, which we substitute in eq65 (where  is the spin analogue of eq61) to give
is the spin analogue of eq61) to give  .
.
Substituting eq164 and  in
in 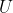 yields
yields

For a 1-electron atom,  and so
and so

Eq260 can be written in terms of the Larmor frequency of the electron. From eq149,  . So,
. So,  . Swapping
. Swapping  with the Thomas precession rate
with the Thomas precession rate  , we obtain the correction term of
, we obtain the correction term of  .
.
The total spin-orbit Hamiltonian is

The spin-orbit energy  corresponds to the expectation value
corresponds to the expectation value  , where
, where 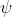 is the hydrogenic wavefunction. Since
is the hydrogenic wavefunction. Since \cdot(\boldsymbol{\mathit{J}}+\boldsymbol{\mathit{S}})=\hat{L}^2+\hat{S}^2+2\boldsymbol{\mathit{J}}\cdot\boldsymbol{\mathit{S}}) , we have
, we have ) . Substituting this and
. Substituting this and  into
into  gives:
gives:
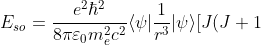-L(L+1)-S(S+1)])
For a given 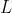 , we usually express the above equation as:
, we usually express the above equation as:
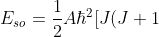-L(L+1)-S(S+1)]\;\;\;\;\;\;\;\;261a)
where  is regarded as a constant.
is regarded as a constant.
For a multi-electron system,

The spin-orbit energy expression for a multi-electron system is very similar to eq261a if we assume Russell-Saunders coupling, where spin-orbit interactions are weak compared to the Coulomb interaction ( ). Here, the electrons’ orbital angular momenta couple to form the total orbital angular momentum
). Here, the electrons’ orbital angular momenta couple to form the total orbital angular momentum  separately from their spin angular momenta, which couple to form
separately from their spin angular momenta, which couple to form  . Each electron in the system experiences a force due to its Coulomb interaction with the other electrons
. Each electron in the system experiences a force due to its Coulomb interaction with the other electrons  .
.
Consider electron 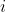 at
at ) along the laboratory
along the laboratory 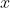 -axis and electron
-axis and electron 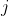 at
at ) on the
on the 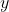 -axis. The Coulomb force
-axis. The Coulomb force  between electrons
between electrons 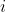 and
and 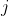 acts in the direction
acts in the direction  , pointing downwards and to the right. The component of
, pointing downwards and to the right. The component of  perpendicular to the
perpendicular to the 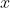 -axis produces a torque
-axis produces a torque  on electron
on electron 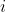 , where
, where  . Because the electrons are not symmetrically arranged at every instant, the resultant torque on electron
. Because the electrons are not symmetrically arranged at every instant, the resultant torque on electron 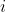 due to all other electrons is generally non-zero. Since
due to all other electrons is generally non-zero. Since  (see eq71), the individual orbital angular momenta are not conserved. By Newton’s third law, the torque exerted by electron
(see eq71), the individual orbital angular momenta are not conserved. By Newton’s third law, the torque exerted by electron 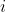 on electron
on electron 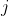 is equal and opposite to the torque exerted by
is equal and opposite to the torque exerted by 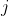 on
on 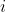 . When summed over all electrons, these internal torques cancel, giving
. When summed over all electrons, these internal torques cancel, giving

Thus, while the total orbital angular momentum  is conserved, the individual
is conserved, the individual  are not. To satisfy
are not. To satisfy  ,
,  and
and  at all times, the individual orbital angular momenta can change only in direction, leading to their precession about the fixed total orbital angular momentum
at all times, the individual orbital angular momenta can change only in direction, leading to their precession about the fixed total orbital angular momentum  . By the same reasoning, the individual spin angular momenta precess around
. By the same reasoning, the individual spin angular momenta precess around  . Since
. Since  , these precessions occur on a much shorter timescale than the subsequent precessions of
, these precessions occur on a much shorter timescale than the subsequent precessions of  and
and  around the total angular momentum
around the total angular momentum  .
.
To continue the derivation of the spin-orbit energy expression for a multi-electron system, we decompose the individual orbital and spin angular momenta into components parallel and perpendicular to  and
and  :
:

Under rapid precession, the time-averaged value of  and
and  vanish. So, the average value of
vanish. So, the average value of  is
is

Since \hat{\boldsymbol{\mathit{L}}}=\biggr\(\boldsymbol{\mathit{L}}_i\cdot\frac{\boldsymbol{\mathit{L}}}{\vert\boldsymbol{\mathit{L}}\vert}\biggr\)\frac{\boldsymbol{\mathit{L}}}{\vert\boldsymbol{\mathit{L}}\vert}=\frac{\boldsymbol{\mathit{L}}_i\cdot\boldsymbol{\mathit{L}}}{L^2}\boldsymbol{\mathit{L}}) and correspondingly
and correspondingly  , where
, where  and
and  are unit vectors,
are unit vectors,

where (\boldsymbol{\mathit{S}}_i\cdot\boldsymbol{\mathit{S}})}{L^2S^2}) .
.
Substituting eq261c into eq261b gives:

It follows that
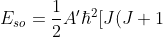-L(L+1)-S(S+1)]\;\;\;\;\;\;\;\;261d)
If spin-orbit interactions become strong compared to the Coulomb interaction ( ),
),  -coupling replaces Russell-Saunders coupling, and the spin-orbit Hamiltonian must be treated in the form of eq261b.
-coupling replaces Russell-Saunders coupling, and the spin-orbit Hamiltonian must be treated in the form of eq261b.
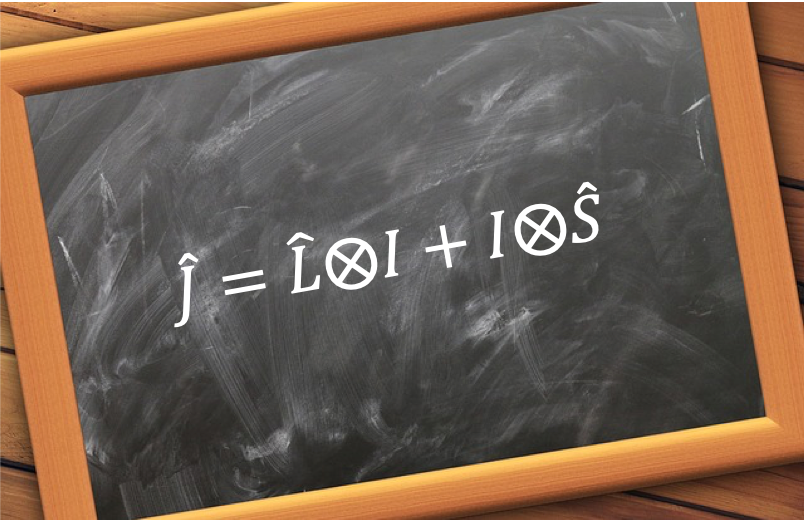
of a 1-electron atom is defined as the vector sum
, where
and
are the orbital angular momentum and spin angular momentum, respectively, of the electron in the atom. For an
-electron atom, Henry Russell and Frederick Saunders proposed that the total angular momentum
of the atom is
, where
and
. If we use a 2-electron atom as an example, the corresponding operator is:
and
. The eigenstates of
are coupled representations of the basis vectors of
and
, while the eigenstates of
are coupled representations of the basis vectors of
and
. The eigenstates of
can either have the form of an uncoupled representation
or a coupled representation
. The uncoupled representation is used when spin-orbit coupling is very weak.
and
are no longer good quantum numbers. In these cases, we switch to
-coupling.

 We shall now show that
We shall now show that