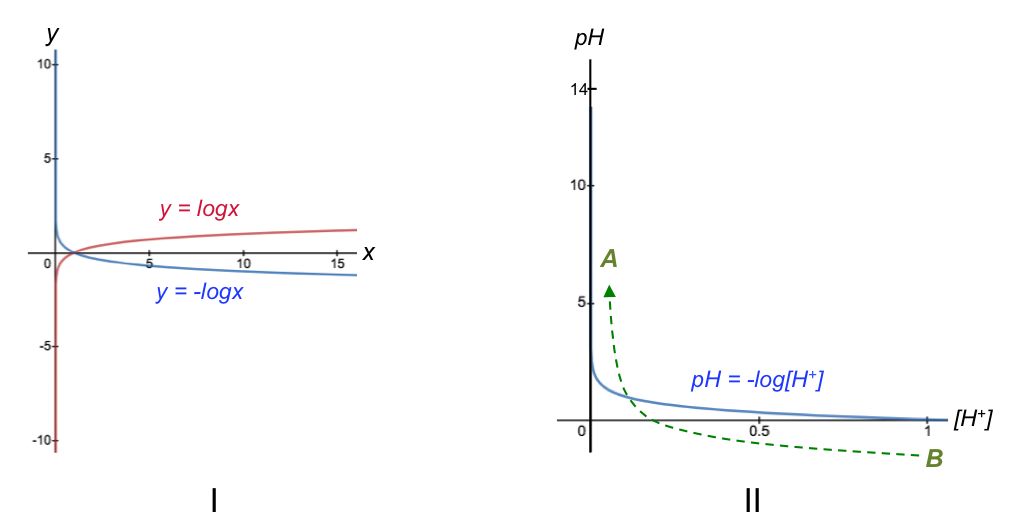
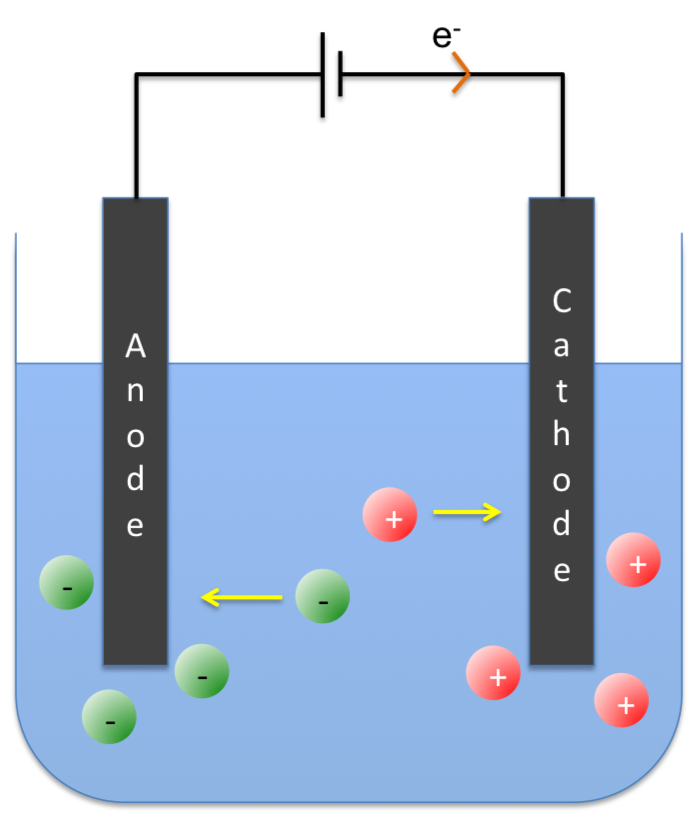
Faraday’s second law of electrolysis states that the amounts of substances produced by the same quantity of electricity in different electrolytes are proportional to their equivalent weights.

In the early 1800s, chemists carried out their research without the knowledge of the mole concept and the molar mass. For example, Joseph Gay-Lussac, a French chemist, discovered in 1805 that two volumes of water vapour were formed by two volumes of hydrogen gas and one volume of oxygen gas. Furthermore, chemists often compared the weights of elements that reacted with one gramme of hydrogen gas and referred to them as equivalent weights, We (i.e., weight equivalent to one gramme of hydrogen gas). Hence, a system of equivalent weights was developed through many experiments. For example,
|
Reaction
|
Element |
Equivalent weight, We (g)*
|
 |
Oxygen
|
8.0 |
 |
Chlorine
|
35.5
|
 |
Magnesium
|
12.2
|
 |
Nitrogen |
4.7
|
* we now know that the equivalent weight of an element is equal to the element’s molar mass divided by its usual valence, i.e. We = M/z.
Faraday experimented on different electrolytes and compared the equivalent weights of certain elements to his results, some of which were similar to the data in the table below:
|
Reaction
|
Electrode
|
Substance liberated
|
Quantity of electricity passed, Q (C)
|
W (g) |
W/We |
 |
Cathode
|
H2
|
1 |
1.04 x 10-5 |
1.04 x 10-5
|
|
Anode
|
O2
|
1 |
8.29 x 10-5 |
1.04 x 10-5
|
 |
Cathode
|
H2
|
1 |
1.04 x 10-5 |
1.04 x 10-5
|
|
Anode
|
Cl2
|
1 |
3.65 x 10-4 |
1.03 x 10-5
|
 |
Cathode
|
H2
|
30,000 |
0.31 |
0.31
|
|
Anode
|
O2
|
30,000 |
2.49 |
0.31
|
 |
Cathode
|
H2
|
30,000 |
0.31 |
0.31
|
|
Anode
|
Cl2
|
30,000 |
11.02 |
0.31
|
 |
Cathode
|
H2
|
50,000 |
0.52 |
0.52
|
|
Anode
|
O2
|
50,000 |
4.15 |
0.52
|
 |
Cathode
|
H2
|
50,000 |
0.52 |
0.52
|
|
Anode
|
Cl2
|
50,000 |
18.37 |
0.52
|
He found that, for a particular quantity of electricity:
)
In other words, W/We is a constant for a particular quantity of electricity, i.e.:
)
where C is the constant of proportionality, which in the example given above is equal to 1.04 x 10-5, 0.31 or 0.52 when the amount of electricity used is 1 C, 30,000 C or 50,000 C, respectively.
Faraday summarized his findings as:
The amounts of substances (W) produced by the same quantity of electricity (Q) in different electrolytes are proportional to their equivalent weights (We)
This is Faraday’s second law of electrolysis.

Comparing eq1 and eq3,
)
We shall now show that the denominator on the RHS of eq4, We/Z, is a constant. We start by rewriting eq2 as:

Substituting eq1 into the numerators of the above equation and noting that the amounts of hydrogen, oxygen and chlorine are deposited at the same Q:

)
Eq5 shows that We,x/Zx is a constant for all x if Faraday’s second law of electrolysis is true, where x represents a particular element. We can therefore rewrite eq4 as C = Q/F, where F = We/Z, and eq3 as:
)
Many years later, the concept of equivalent weights was superceded by the concept of molar mass and a modern version of eq6 is:
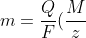)
W is replaced with m, the mass of the substance formed, and We with M/z, where M is the molar mass of the electrolysed ion and z is the valency of the electrolysed ion.
The value of F was subsequently refined to 96,485.3329 Cmol-1 (now known as one mole of electric charge) and named the Faraday constant, in honour of the scientist.