The construction of term symbols in the spin-orbit coupled representation (i.e. levels) is an extension of the procedure for deriving term symbols in the spin-orbit uncoupled representation (i.e. terms).
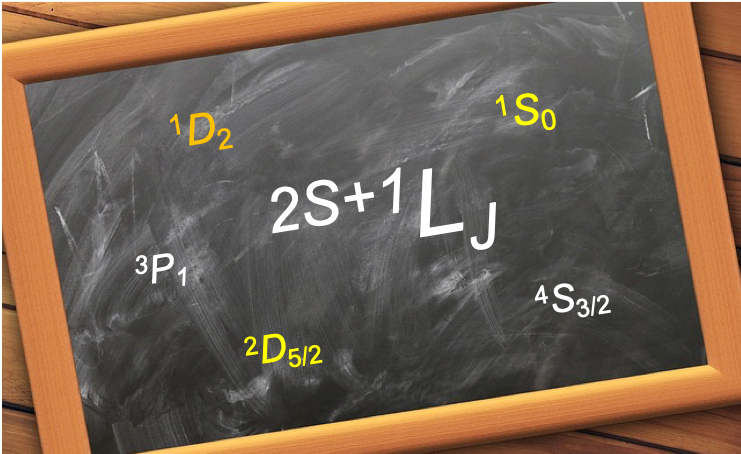
Russell-Saunders (LS) coupling
For the p2 configuration of the carbon atom, we have shown in the previous article that the terms are 1D, 3P and 1S with degeneracies of 5, 9 and 1 respectively. The total degeneracy of 15 corresponds to 15 microstates in the uncoupled representation, which are the basis states for the coupled representation. Since the total number of microstates describing a system is independent of the chosen representation, we have 15 coupled states after linearly combining the uncoupled basis states. The procedure to construct levels is to use the Clebsch-Gordan series, which describes the allow values of the total angular momentum number
for a given value of
and a given value of
, while ensuring that the total number of microstates (degeneracy) of each term (e.g. 3P) is equal to the total number of microstates of the associated levels (e.g. 3P2, 3P1, 3P0), i.e.
.
|
Term |
Degeneracy | Level |
Degeneracy |
|
|
1D |
5 | 2 = 2 + 0 | 1D2 |
5 |
|
3P |
9 |
2 = 1 + 1 | 3P2 | 5 |
| 3P1 |
3 |
|||
|
3P0 |
1 | |||
| 1S | 1 | 0 = 0 + 0 | 1S0 |
1 |
For the p3 configuration of nitrogen, the terms are 4S, 2D, 2P and the levels are:
|
Term |
Degeneracy | Level | Degeneracy | |
| 4S | 4 | 3/2 = 0 + 3/2 | 4S3/2 |
4 |
|
2D |
10 | 5/2 = 2 + 1/2 | 2D5/2 | 6 |
| 2D3/2 |
4 |
|||
|
2P |
6 | 3/2 = 1 + 1/2 | 2P3/2 | 4 |
| 2P1/2 |
2 |
You’ll notice that the degeneracies for both carbon and nitrogen are not fully lifted (i.e. each level may contain more than one state) when spin-orbit interactions are considered. Other effects, like an external magnetic field, are required to completed lift the degeneracy of an atom.
jj-coupling
In jj-coupling, each electron of the p2 configuration has and
, which combine to give
or
. For each value of
, there are
allowed
values (see this article for details). Thus, each electron is described by the one-electron wavefunctions
,
,
,
,
and
, giving a total of six one-electron states.
According to the Pauli exclusion principle, no two electrons can can be described by the same set of quantum numbers. Therefore, two-electron wavefunctions like are forbidden.
The 15 Pauli-allowed microstates can be grouped as follows:
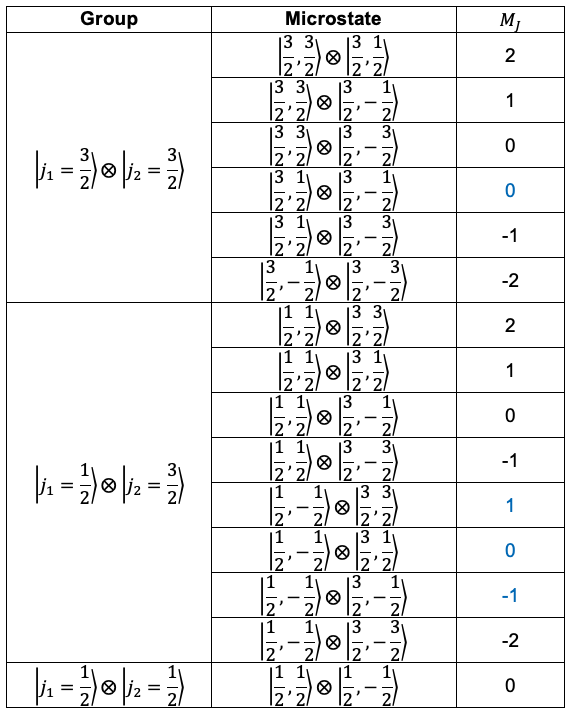
For the group, the microstates with
spanning from +2 to -2 form the term
, with the remaining microstate with
belonging to
. Similarly, for the
group, the microstates correspond to the terms
and
. Therefore, the jj-coupling term symbols for the p2 configuration are
,
,
,
and
.