A fuel cell converts chemical energy from the fuel (usually H2) to electrical energy, which is used to power engines. With reference to the diagram below, H2 is fed into the left compartment, where it is oxidised to H+ at the anode, which is porous and impregnated with a Pt catalyst. The protons then migrate across the electrolyte (H3PO4) contained in a polymer exchange membrane that only allows the passage of H+.
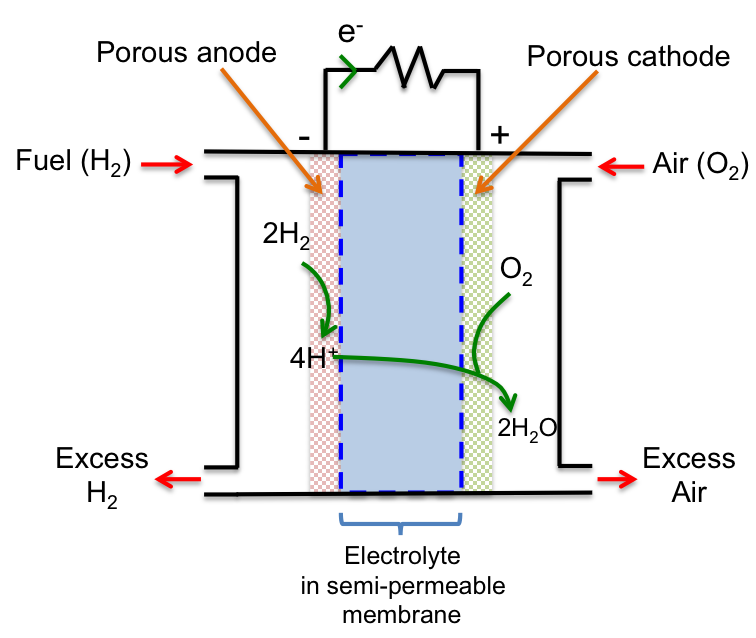
At the cathode, O2 in the air fed into the right compartment reacts with H+ and is reduced to form water. The cathode is again porous and impregnated with a Ni catalyst. The overall redox reaction is:
2H2 (g) + O2 (g) → 2H2O (l)
next article: The alkaline battery
Previous article: Other forms of electrochemical cell
Content page of basic electrochemistry
Content page of Basic chemistry
Main content page