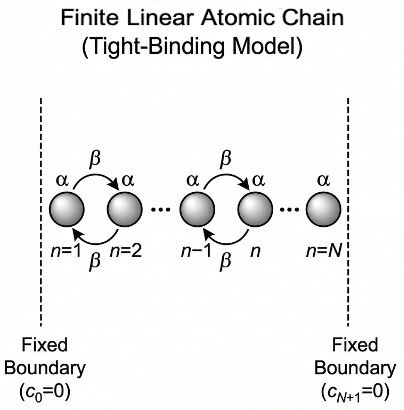
The tight-binding model incorporates the Hückel approximation to describe the electronic structure of solids by treating electrons as localised around atoms and hopping between neighbouring sites.
Consider a linear chain of identical atoms located at position
, with
representing the s-orbital of atom
. According to the Hückel approximation, the Hamiltonian
is defined by two parameters:
1) is the energy of an electron localised on a single atom.
2) is the energy associated with an electron hopping between adjacent atoms.
3) All other matrix elements are zero.
To find the eigenvalues and eigenstates
satisfying the Schrödinger equation
, we adopt the linear combination of atomic orbitals (LCAO) approach, in which
Multiplying the Schrödinger equation on the left by the bra gives:
Substituting eq1 into eq2, and noting that the states are orthonormal, yields:
Expanding the first summation in eq3 and applying the Hückel approximation results in:
Substituting the trial solution into eq4, and imposing the boundary conditions of
, gives:
This rearranges to:
Using the trigonometric identity , with
and
yields:
which simplifies to:
Since , we have
. So,
, which when substituted into eq5 gives:
where .
Although is an integer due to the condition
, its specific range
in eq6 follows the fact that each eigenvalue
is associated with one of
linearly independent eigenstates
described by eq1.

Question
Show that and
are trivial solutions.
Answer
If , then
and
everywhere, which is a trivial solution. If
, then
and
is again zero everywhere. Therefore, the non-trivial solutions correspond to
.
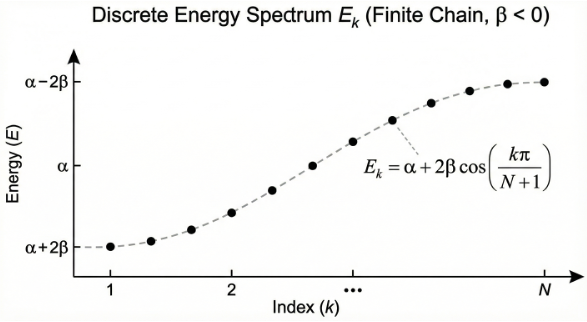
An electron localised on an atom resides in a bound state with energy measured relative to the vacuum level (). Therefore, the conventional value of
is negative. For s-orbitals,
is also negative because
corresponds to an integral of the form (positive wavefunction)
(negative potential)
(positive wavefunction). It follows that the lower-energy states occurs when
is small in eq6 (so that the cosine term is close to +1 for large
). In other words,
and
represent the lowest and highest energy states of the system respectively (see diagram below).
One important application of the tight-binding model is its role in explaining band theory, which will be explored in the next article.