What are the different definitions of acids and bases?

Svante Arrhenius (in 1884)
Arrhenius acid: A substance, when in its aqueous state, produces hydrogen ions, H+.
Arrhenius base: A substance, when in its aqueous state, produces hydroxide ions, OH–.
For example, aqueous hydrogen chloride (or hydrochloric acid) is an acid:
However, these definitions are restrictive, for example, they do not consider gaseous hydrogen chloride and gaseous ammonia as an acid and a base respectively.
Bronsted-Lowry (in 1923)
Bronsted acid: A substance that is a H+ (proton) donor.
Bronsted base: A substance that is a H+ acceptor.
Bronsted-Lowry’s definitions expand on Arrhenius’. Gaseous hydrogen chloride and gaseous ammonia are now an acid and a base respectively.
Gaseous hydrogen chloride donates a proton to water to form the hydronium ion, H3O+ while gaseous ammonia accepts a proton from water to form the ammonium ion, NH4+. Note that the hydrogen ion is more likely to exist as a hydronium ion (or larger complexes like H9O4+) in water than as an isolated proton, H+. The hydronium and ammonium ions formed are acids too as they can donate their protons to bases. Bronsted and Lowry called them conjugate acids. Similarly, the chloride and hydroxide ions are bases as they can accept protons from acids. They are called conjugate bases. In general,
The HA/A– and BH+/B pairs are called conjugate acid-base pairs.

Question
Is OH– in the following reaction a base or an acid?
Answer
O2- is a base and H2O is an acid. OH– is a conjugate acid of O2- and a conjugate base of H2O.
Acids with one, two and three dissociable hydrogen atoms are known as monoprotic (e.g. HCl), diprotic (e.g. H2SO4) and triprotic (e.g. H3PO4) respectively. Acids with more than one dissociable hydrogen atoms are collective called polyprotic acids. Similarly, bases that can accept one, two and many protons are called monoprotic bases, diprotic bases and polyprotic bases respectively. Finally, some substances can be both proton acceptors and proton donors, for example, the hydrogen carbonate ion, HCO3– :
Such substances are called amphiprotic.
Gilbert Lewis (in 1938)
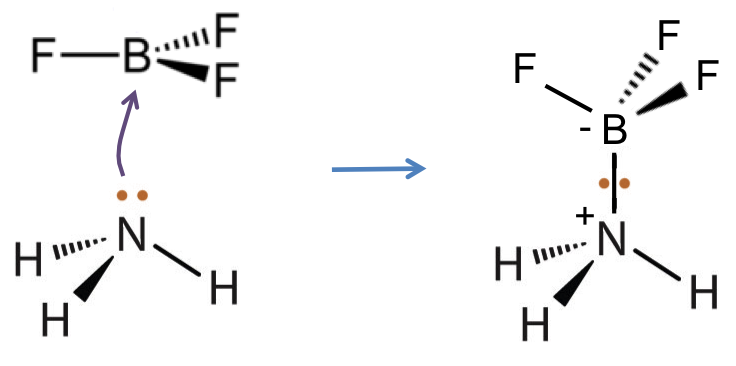
Lewis acid: A substance that is an electron pair acceptor.
Lewis base: A substance that is an electron pair donor.
According to Lewis’ definitions, HCl is an acid and NH3 is a base because HCl accepts a pair of electrons from the nitrogen atom of NH3 (the electron pair donor) to give the ammonium and chloride ions.

Lewis’ definitions not only encompass substances under Bronsted-Lowry’s definitions but also include species that do no H, e.g. BF3.

Question
What is the difference between a base and an alkali?
Answer
An alkali is a hydroxide of a group 1 (alkali metal) or group 2 (alkaline earth metal) element with pH > 7 in its aqueous form, e.g. NaOH and Mg(OH)2. A base is defined either by an Arrhenius base, a Bronsted base or a Lewis base. In other words, an alkali is a soluble hydroxide, while a base can be any compound that fits the definition of either an Arrhenius base, a Bronsted base or a Lewis base, e.g. ammonia gas, NaH and any soluble hydroxide. Therefore, we can also define an alkali as the solution of a water-soluble base.
