The pH scale is a range of numbers for measuring the extent of acidity or basicity of a substance.
As mentioned in the previous article, pH is defined as –log[H+] and is accurate over the range of 0 to 14, which is equivalent to concentrations of aqueous H+ of between 1 M to 10-14 M. We can use the values of 0 to 14 to construct a colour-coded pH scale as a convenient reference of the concentration of H+ in solutions.
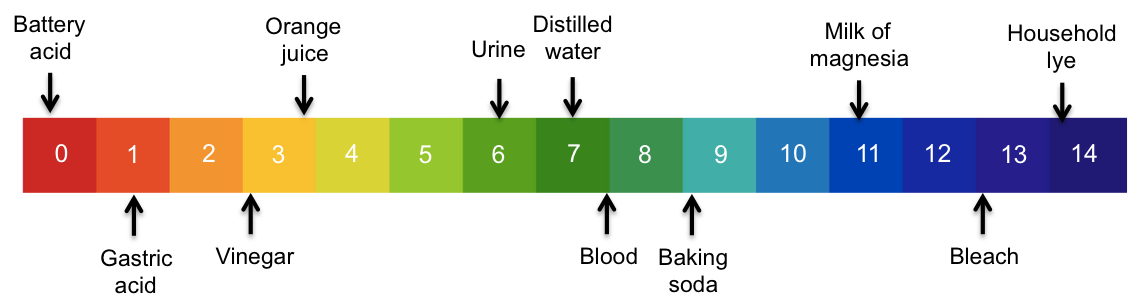
So, instead of saying that the concentration of H+ in a sample of urine is 10-6 mol dm-3, we simply say that the sample of urine has a pH of 6.

Question
What if the concentration of H+ in a solution is 2 M? Does it mean that the pH of the solution is -0.3?
Answer
We often use a pH glass electrode to measure the pH of an aqueous solution. Although it is not wrong to say that the pH of a 2 M HCl solution is -0.3, the pH scale becomes less accurate when the concentration of a solution is greater than 1 M. This is because acid molecules may be absorbed by a layer of gel, which is coated on the glass bulb (see below diagram), lowering the activity of H+ at the electrode, making it harder to accurately measure the real concentration of H+ in the solution. On the other hand, a solution with pH < 10-14 M has so low a concentration of H+ that other ions present in the solution may be detected by the electrode instead, giving an inaccurate reading. This is why the pH scale is usually quoted between 0 to 14. Fortunately, many common solutions like those stated in the diagram above have pH values within the measurable range.

