Anodising is an electrolytic process to create an inert oxide layer on a metal, which is the anode and hence the name anodising. The diagram below shows the passivation of aluminium by the formation of a layer of aluminium oxide, which protects the aluminium from corrosion. Anodised aluminium is used in many products, e.g. aircraft parts, consumer electronic components, carabineers, etc.
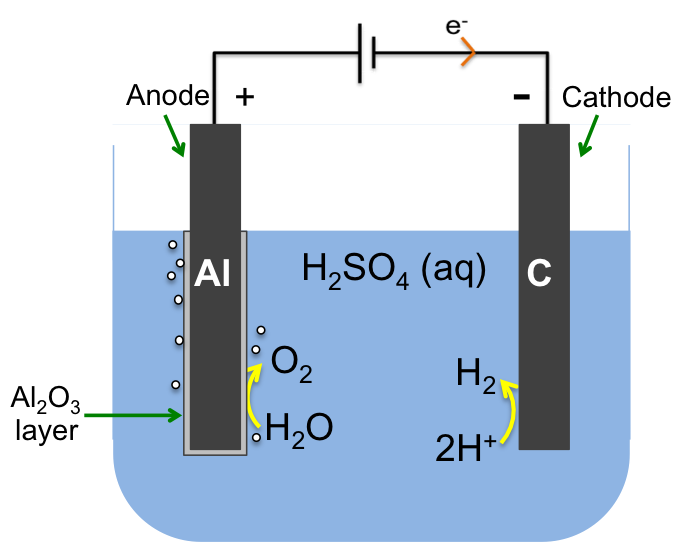
At the anode:
2H2O (l) → O2 (g) + 4H+ (aq)+ 4e–
The oxygen produced reacts with the anode to form the protective layer:
3O2 (g) + 4Al (s) → 2Al2O3 (s)
