Electrolytic cell 4 in the earlier question is an example of the use of electrolysis to refine metals. Copper ore, which contains small amounts of other metals like Pt and Au, is the anode, while a piece of pure Cu is the cathode.
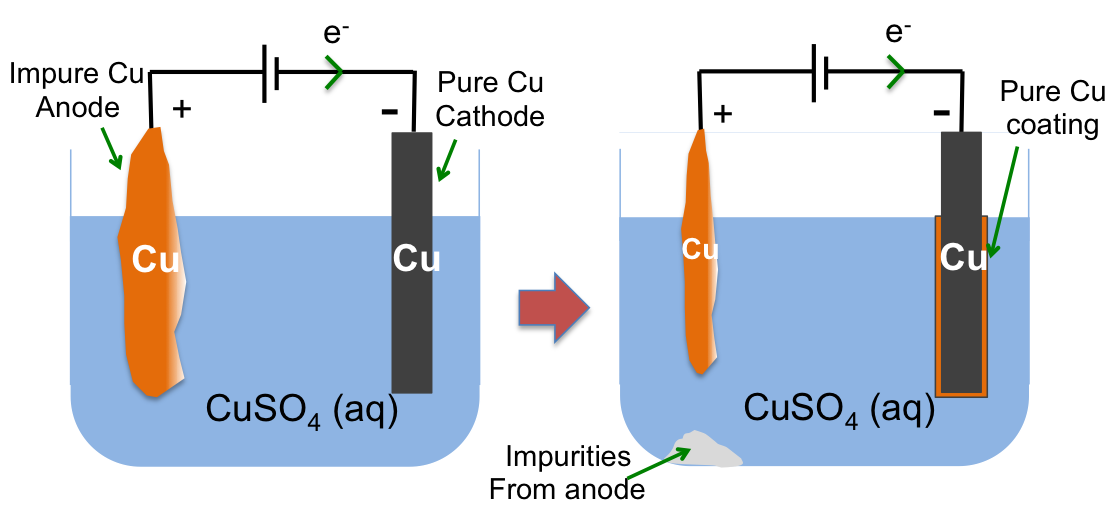
At the anode, Cu, which is competing with H2O and SO42-, is preferably oxidised to aqueous Cu2+ as it lies above H2O and SO42- in the electrochemical series and is rather concentrated relative to the other two species (i.e. high number of Cu atoms in the electrode per unit volume as compared to the other two species in the electrolyte per unit volume).
Cu (s) →Cu2+ (aq) + 2e–
At the cathode, Cu2+, which is competing with H2O, is preferably reduced to Cu as it is lower in the electrochemical series than H2O and is again, rather concentrated relative to H2O.
Cu2+ (aq) + 2e– → Cu (s)
The overall redox reaction results in the coating of a layer of pure copper on the surface of the pure copper cathode:
Cu (s) → Cu (s)
If we replace the anode with a pure metal, e.g. Ni, and the electrolyte with an aqueous salt of the metal, e.g. NiSO4, a layer of Ni will coat the cathode (see diagram below). This is the principle of electroplating.
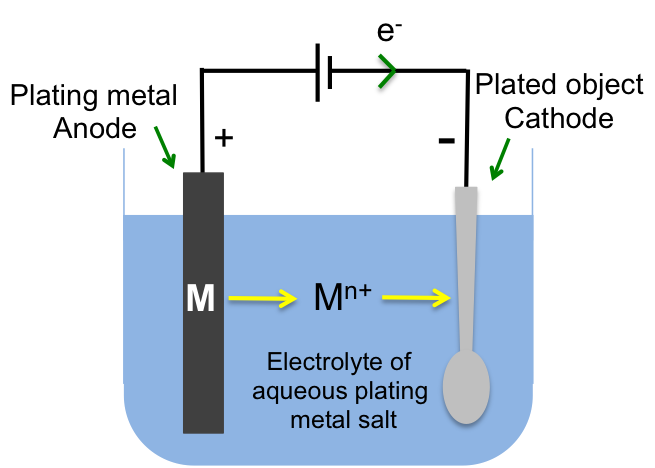
Examples of electroplating are:
|
Plating Metal, M |
Plated object |
|
Ni, Cr |
Automotive parts, electrical appliances |
|
Sn |
Ornaments, jewelry |
|
Ag, Au, Cu |
Cutlery, ornaments, jewelry, electronic components |