A three-electrode cell is used for electrochemical measurements involving the passage of current. It is a good set-up to evaluate electrochemical quantities like overpotential and IR drop. A typical three-electrode system consists of a working electrode, a reference electrode and a counter (or auxillary) electrode (see diagram below).
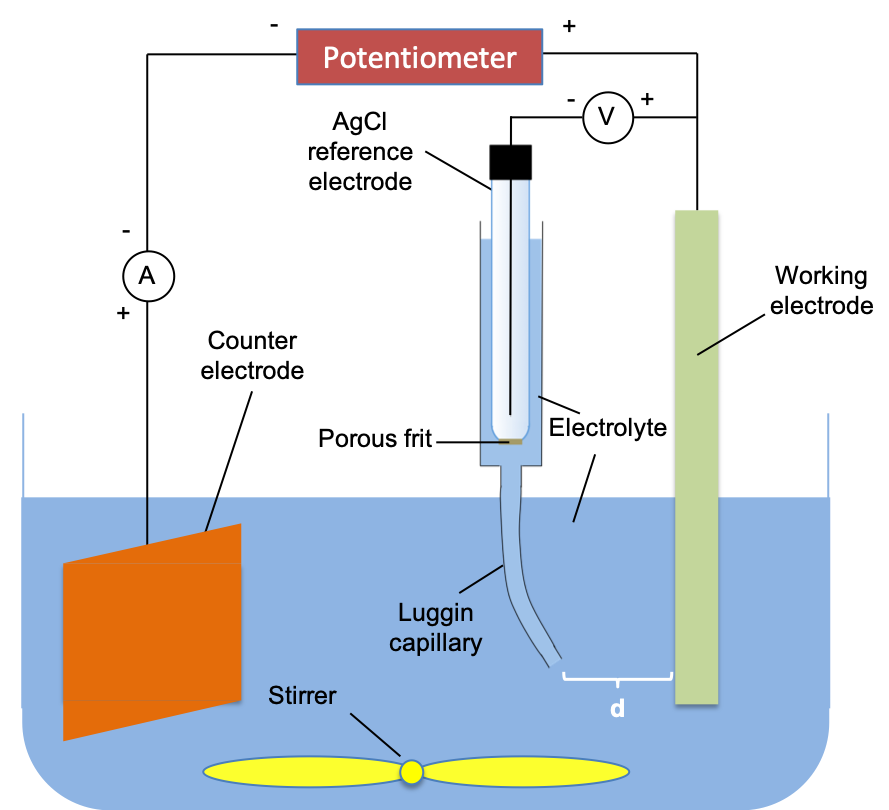
Generally, the potential of a reference electrode must remain constant in order to measure the potential of a working electrode. However, electrodes become polarised when a current flows. The solution is to introduce a third electrode called a counter electrode to the cell for dynamic measurements. A high impedance voltmeter displays the potential of the working electrode and is connected between the working electrode and the reference electrode so that very negligible current flows between them. Experiments involving the polarisation of the working electrode at various current levels can therefore be conducted with respect to the counter electrode. Furthermore, the reference electrode is fitted with a movable column of electrolyte called the Luggin capillary, which allows the measurement of IR drop by varying the distance d.
It is important to note that the counter electrode serves only to complete the circuit for current flow. Ideally, it should not to interfere with reaction at the working electrode and is designed to be non-polarisable. One way to minimise the formation of overpotential at the counter electrode is to increase its surface area, A. This in turn reduces its current density (a fixed amount of current from the potentiostat is spread over a larger area), which is described by the Butler-Volmer equation:
Since j0 is an intensive property, is small if A is large. This implies that
Since f ≠ 0, η ≈ 0.
