Reactions occur when particles collide with sufficient energy to overcome the potential energy of repulsion between the electron densities of the approaching particles, and often, to break and rearrange bonds in the particles.

Therefore, not all collisions successfully result in a reaction. For a reaction to take place, particles must collide with the correct orientation and a minimum amount of energy called the activation energy, Ea, which differs between reactions.
Consider the collision of dinitrogen tetraoxide molecules to form nitrogen dioxide.
A sample of at a particular temperature consists of molecules with various energies. This is because after colliding with one another, some molecules gain energy while others lose energy, with the bulk of the molecules having intermediate energies. A fraction, however, have sufficient energy, ≥ Ea, to collide with each other in the correct orientation and dissociate into nitrogen dioxide molecules. Such collisions are called effective collisions.
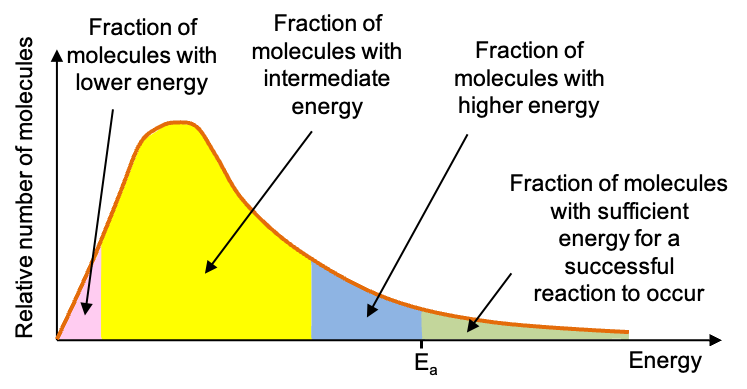
The different energies of the molecules can be described by the graph above. The graph, called the Maxwell-Boltzmann distribution, was first derived by James Maxwell, a Scottish scientist in 1860 and later improved upon by Ludwig Boltzmann, an Austrian physicist. The various areas under the curve represent the fraction of molecules with certain energies, with the green area expressing the proportion of molecules with sufficient energies to react.
In general, factors that increase the rate of effective collisions between particles, or provide alternate reaction pathways with lower activation energies, will increase the rate of the reaction. These factors are:
-
- the concentration of the reactants (or pressure, if the reactants are gases)
- the surface area of the reacting particles
- the temperature at which the reaction is occurring, and
- the presence of a catalyst.