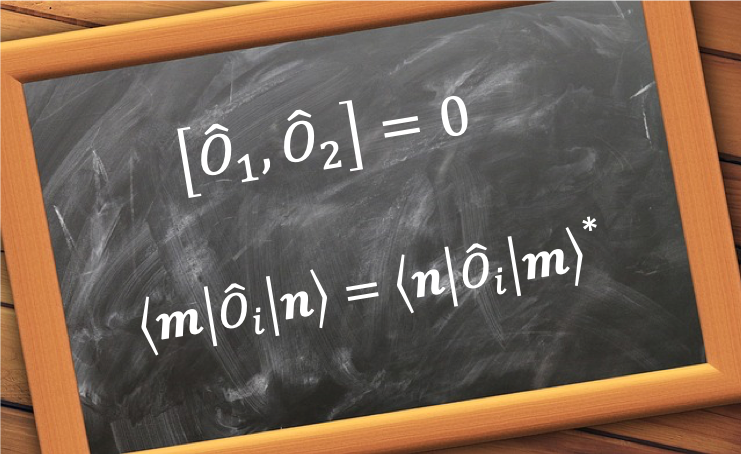A linear combination of wavefunctions is the weighted sum of a complete set of basis wavefunctions .
It is commonly used as a technique in quantum chemistry to approximate a multi-electron wavefunction , where
Because the Hamiltonian operator is a linear operator, a linear combination of
is a solution to
if
are solutions to
. However, it is only meaningful to solve the eigenvalue equation
, not just finding solutions to
. The problem is
(e.g.
) is not an eigenfunction of
:
The terms on the RHS of the 2nd equality of the above equation cannot be expressed as , which implies that a state described by
does not have a well-defined eigenvalue. Nevertheless, we can find the average value (or expectation value) of
. This means that for wavefunctions that are not eigenfunctions of
, we can use approximations like
to find
. The exception to eq42 is if all the basis functions describe a degenerate eigenstate, where
:
In general, a linear combination of wavefunctions (or a linear combination of atomic orbitals, LCAO) is a method of finding an easy or solvable solution to an otherwise complicated or unsolvable one. LCAO is often used in:
-
- Finding the energy of a system using the Hartree-Fock-Roothan method.
- Eliminating complex wave functions to find the formula of hydrogenic p/d/f-orbitals that describe their corresponding p/d/f degenerate states.
- Constructing wavefunctions that satisfy Pauli’s exclusion principle.
- Explaining the relationship between symmetry and degeneracy in group theory.
- Constructing symmetry-adapted linear combinations (SALCs) of orbitals, which are building blocks of a molecular orbital (MO) of a molecule.