The ‘initial rate proportional to 1/t’ concept is applicable to a chemical kinetics experiment that has the initial concentration of a reactant varied but the measured amount of a product fixed. One such experiment is the decomposition of hydrogen peroxide over a platinum catalyst, while another is the iodine clock. For the decomposition experiment, we have:
\rightleftharpoons2H_2O(l)+O_2(g))
Using various initial concentrations of H2O2, different times taken to collect a fixed volume of oxygen are recorded. The diagram below illustrates the setup, where the fixed volume of oxygen produced under constant temperature is measured by a fixed change in pressure of the constant-volume system.
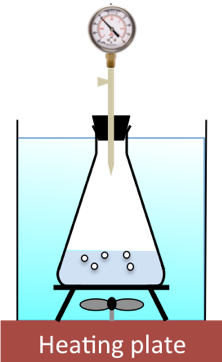
Since the reaction is very slow, we assume that the initial concentration of H2O2 for each experiment remains relatively unchanged for the volume of oxygen generated (the change in pressure of the system can be fixed at an amount that does not exceed three minutes to occur). Using eq10, and assuming gases behave ideally, we can write
![initial\, rate=\frac{d[O_2]}{dt}=\frac{\Delta (n_{O_2}/V)}{\Delta t}=\frac{\Delta p}{RT\Delta t}\; \; \; \; \; \; \;\; 27](https://latex.codecogs.com/gif.latex?initial\,&space;rate=\frac{d[O_2]}{dt}=\frac{\Delta&space;(n_{O_2}/V)}{\Delta&space;t}=\frac{\Delta&space;p}{RT\Delta&space;t}\;&space;\;&space;\;&space;\;&space;\;&space;\;&space;\;\;&space;27)
To produce a fixed volume of oxygen, the change in amount of oxygen, and hence the change in pressure of the system, is constant regardless of the concentration of H2O2 used. Thus,

where C is a constant and is equal to  .
.
This answers the question of why initial rate is proportional to 1/t.
To develop an equation that that allows us to further analyse the decomposition reaction, we propose the following rate law:
![initial\, rate=k[H_2O_2]_0^{\;\: i}\; \; \; \; \; \; \; \; 29](https://latex.codecogs.com/gif.latex?initial\,&space;rate=k[H_2O_2]_0^{\;\:&space;i}\;&space;\;&space;\;&space;\;&space;\;&space;\;&space;\;&space;\;&space;29)
where [H2O2]0 is the initial concentration of H2O2.
Taking the natural logarithm for both sides of eq28 and eq29, we have
=ln\frac{1}{\Delta&space;t}+ln\frac{\Delta&space;p}{RT}\;&space;\;&space;\;&space;\;&space;\;&space;\;&space;\;&space;\;&space;30)
and
![ln(initial\, rate)=iln[H_2O_2]_0+lnk\; \; \; \; \; \; \; \; 31](https://latex.codecogs.com/gif.latex?ln(initial\,&space;rate)=iln[H_2O_2]_0+lnk\;&space;\;&space;\;&space;\;&space;\;&space;\;&space;\;&space;\;&space;31)
Substitute eq31 in eq30
![ln\frac{1}{\Delta t}=iln[H_2O_2]_0+ln\frac{k}{\Delta p/RT}\; \; \; \; \; \; \; \; 32](https://latex.codecogs.com/gif.latex?ln\frac{1}{\Delta&space;t}=iln[H_2O_2]_0+ln\frac{k}{\Delta&space;p/RT}\;&space;\;&space;\;&space;\;&space;\;&space;\;&space;\;&space;\;&space;32)
Therefore, using the time measurements for the fixed change in pressure to occur for different initial concentrations of H2O2, a plot of  versus
versus ![ln[H_2O_2]_0](https://latex.codecogs.com/gif.latex?ln[H_2O_2]_0) gives a gradient of the order of the reaction, i, and a vertical intercept of
gives a gradient of the order of the reaction, i, and a vertical intercept of  where k is the rate constant.
where k is the rate constant.


Question
Eq32 is derived using the rate equation ![\frac{d[O_2]}{dt}=k[H_2O_2]_0^{\; \, i}](https://latex.codecogs.com/gif.latex?\frac{d[O_2]}{dt}=k[H_2O_2]_0^{\;&space;\,&space;i}) . What if we define the rate equation as
. What if we define the rate equation as ![-\frac{1}{2}\frac{d[H_2O_2]}{dt}=k_1[H_2O_2]_0^{\; \, i}](https://latex.codecogs.com/gif.latex?-\frac{1}{2}\frac{d[H_2O_2]}{dt}=k_1[H_2O_2]_0^{\;&space;\,&space;i}) ?
?
Answer
![-\frac{1}{2}\frac{\Delta n_{H_2O_2}/V_{H_2O_2}}{\Delta t}=k_1[H_2O_2]_0^{\: \, i}](https://latex.codecogs.com/gif.latex?-\frac{1}{2}\frac{\Delta&space;n_{H_2O_2}/V_{H_2O_2}}{\Delta&space;t}=k_1[H_2O_2]_0^{\:&space;\,&space;i})
![ln\left ( -\frac{1}{2}\frac{\Delta n_{H_2O_2}}{V_{H_2O_2}} \right )+ln\frac{1}{\Delta t}=lnk_1+iln[H_2O_2]_0](https://latex.codecogs.com/gif.latex?ln\left&space;(&space;-\frac{1}{2}\frac{\Delta&space;n_{H_2O_2}}{V_{H_2O_2}}&space;\right&space;)+ln\frac{1}{\Delta&space;t}=lnk_1+iln[H_2O_2]_0)
![ln\frac{1}{\Delta t}=iln[H_2O_2]_0+ln\left ( \frac{k_1}{-\Delta n_{H_2O_2}/2V_{H_2O_2}} \right )\; \; \; \; \; \; \; \; 33](https://latex.codecogs.com/gif.latex?ln\frac{1}{\Delta&space;t}=iln[H_2O_2]_0+ln\left&space;(&space;\frac{k_1}{-\Delta&space;n_{H_2O_2}/2V_{H_2O_2}}&space;\right&space;)\;&space;\;&space;\;&space;\;&space;\;&space;\;&space;\;&space;\;&space;33)
To produce a fixed volume of oxygen, the change in amount of H2O2,  , is a constant value regardless of the initial amounts of H2O2. For example, to produce 1 mole of oxygen, the change in amount of H2O2 is always -2 moles irrespective of how many moles of H2O2 there are to begin with. Furthermore, the same volume of H2O2 is used as we vary [H2O2]. So, the 2nd term on RHS of eq33 is a constant, which makes eq33, like eq32, a linear function.
, is a constant value regardless of the initial amounts of H2O2. For example, to produce 1 mole of oxygen, the change in amount of H2O2 is always -2 moles irrespective of how many moles of H2O2 there are to begin with. Furthermore, the same volume of H2O2 is used as we vary [H2O2]. So, the 2nd term on RHS of eq33 is a constant, which makes eq33, like eq32, a linear function.
Comparing eq33 with eq32,
















