What is the formula of the titration curve of a polyprotic acid versus a monoprotic strong base?
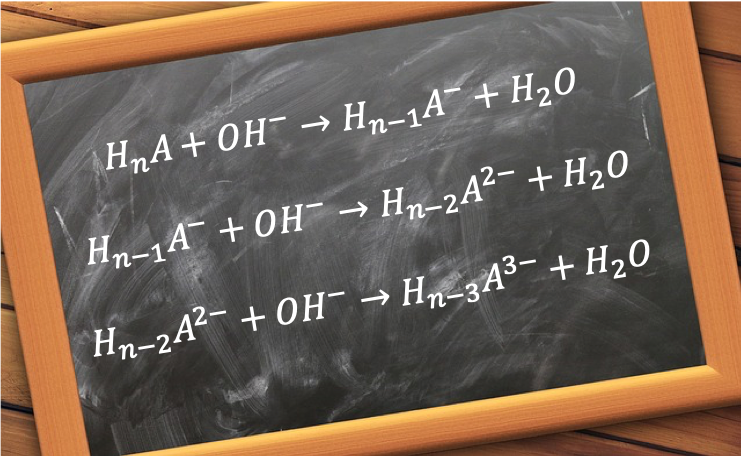
Comparing eq8, eq19 and eq30 the general equation for a polyprotic acid versus a monoprotic strong base titration is:
where n is the basicity of the acid, i.e. the number of replaceable hydrogen atoms in one molecule of the acid.