A pH glass electrode is an ion-selective electrode that is sensitive to H+, and therefore, used to measure pH of solutions. It is composed of a glass bulb that is H+-selective and an internal AgCl electrode.

The glass bulb is specially manufactured with a composition of 70+% SiO2 and 20+% Na2O. It has a silicate network within the glass matrix, with some Si atoms singly bonded to O atoms and other Si atoms coordinated with Na+ (see diagram above). On the outer and inner surfaces of the bulb, some of the Si–O– arms are also coordinated with Na+, while the rest of the Si–O– are uncoordinated.
Before the pH glass electrode can be used, it must be hydrated (soaked in water of pH = 7) to form two layers of gel. This is to allow H+ to diffuse into the gel layers to convert the Si–O–Na+ groups on both surfaces to Si–O–H+. Since the inner surface of the bulb is already in contact with a KCl buffer solution at pH 7, both the inner and outer surfaces develop the same ion-exchange equilibrium when the electrode is dipped in water:
This ion-exchange occurs because H+ binds more strongly to SiO– than Na+. Therefore, the position of the equilibrium is almost entirely to the right. On the inner surface, the displaced sodium ions enter the buffer solution, and on the outer surface, displaced sodium ions enter water. The hydrated surfaces now consist of a combination of SiO–H+ and SiO– arms, and is ready for use. The electrode is immersed in an analyte of unknown pH, establishing the following equilibrium on the outer surface:
and the following equilibrium on the inner surface equilibrium:
If the pH of the analyte is less than 7, the outer surface will have fewer uncoordinated Si–O– arms and hence, less negative charges as compared to the inner wall, whose equilibrium remains unchanged. The difference in charges on the surfaces produces a potential difference across the glass bulb. Furthermore, it confers enough activation energy for sodium ions within the glass matrix to dissociate from the silicate network. These mobile sodium ions then migrate interstitially across the bulb and along the potential gradient, to complete the circuit.
Even though the above equilibria are ion-exchanges and not redox reactions, the potential across the bulb Eb is found empirically to be Nernst-like, where:

Question
Why Eb = Eouter – Einner and not Eb = Einner – Eouter?
Answer
We write Eb = Eouter – Einner to be consistent with the way we write Ecell = ER – EL, which follows the IUPAC convention (consistency is best visualised by tracing the direction of electron flow in the circuit).
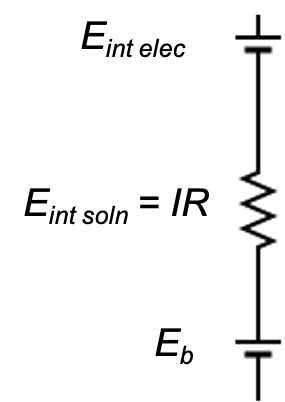
Comparing with a circuit diagram (see above diagram), the overall potential of the glass electrode is:
where Eint elec is the potential of the internal AgCl electrode and Eint soln is the potential drop across the saturated KCl solution.
Since no current flows in potentiometric methods,
Furthermore, manufacturing defect or damage of electrode over time results in the electrode recording a non-zero potential when the analyte is pH = 7. We call this an asymmetry potential, which must be included in Eglass:
Substituting eq28 in eq29, where ainner, Easym and Eint elec are constants, Eglass eletrode at rtp is:
where L = Eint elec + Easym – 0.0592 log ainner.

Question
What is the function of the internal AgCl electrode?
Answer
For the pH glass electrode to work in a pH measurement experiment, there must be 2 electrodes, where redox reactions occur (to generate and receive electrons). The internal AgCl electrode serves as one of the electrodes, while the reference electrode (an external regular AgCl electrode) serves as the other electrode. The bulb, which is the pH-sensing element, can be perceived as an extension of the internal electrode.
