A typical experiment setup for pH measurement consists of a glass electrode (indicator electrode), a reference electrode (a regular AgCl electrode), the analyte and a potentiometer. The potentiometer ensures that current flow is negligible in the electrochemical circuit so that IR drop and overpotential are minimised, resulting in more accurate measurements. Such a method in electrochemistry where current is negligible is called potentiometry.
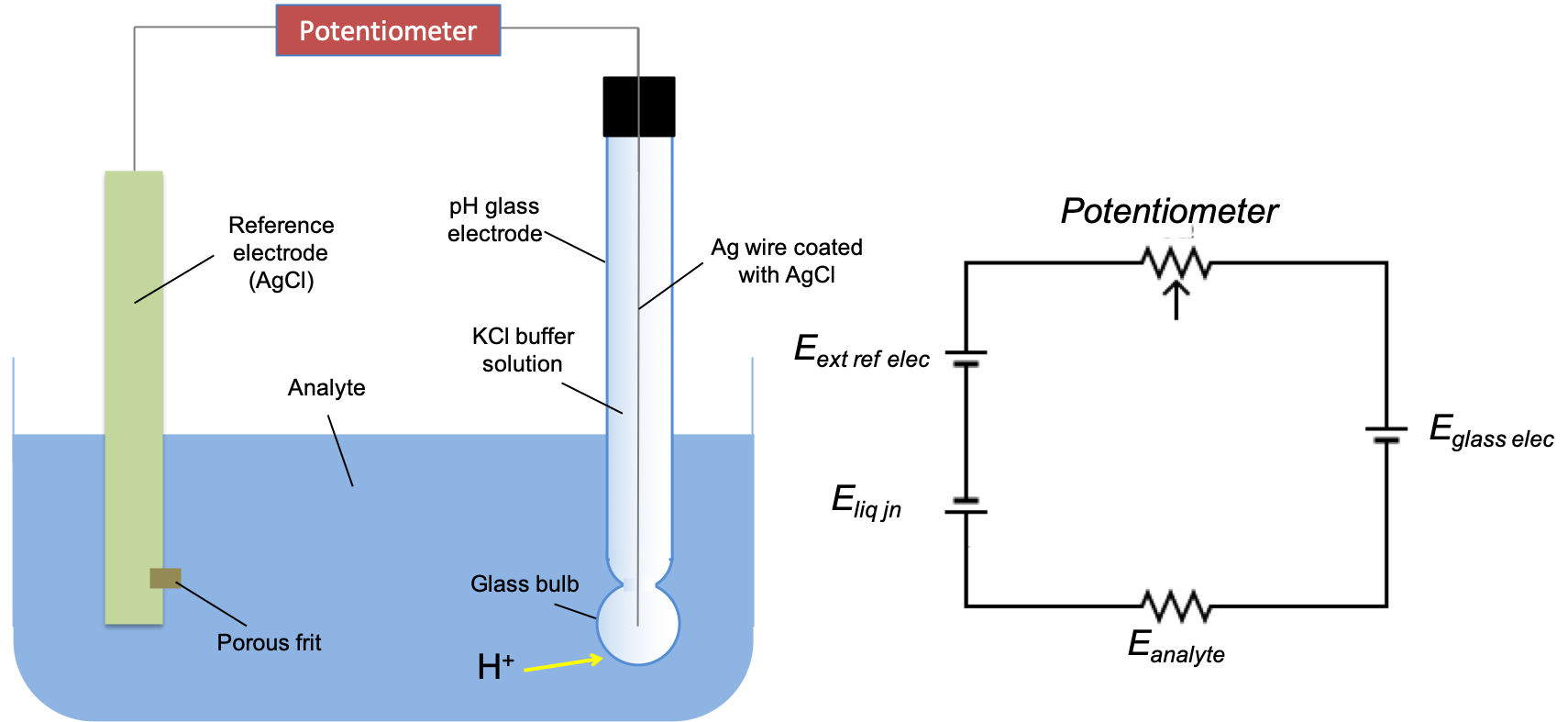
Comparing the setup with a circuit diagram (see above diagram), the overall potential of the electrochemical cell is:
The liquid junction potential arises due to the difference in mobility of K+ and Cl– in the controlled flow of the saturated KCl solution out of the porous frit of the external reference AgCl electrode (to complete the circuit). Eanalyte = IR = 0 since current flow is negligible in potentiometric methods.

Question
How does the potentiometer control current in the circuit?
Answer
A potentiometer works by sliding a contact along a resistor and finding the relationship between VL and Ecell (see diagram below).
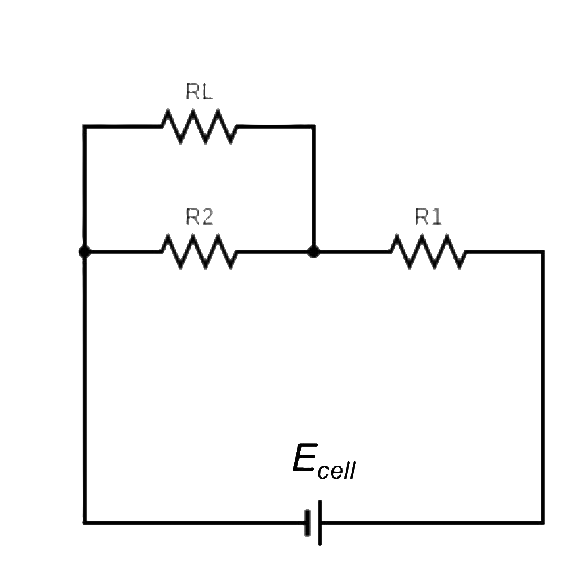
For a pH measurement experiment, the contact moves to the point where no current flows. Ecell is then calculated by:
In a modern pH meter, a computer controls the potentiometer.
Lastly, before a pH meter is put to use, it is calibrated using solutions of known pH. The reason for this is as follows:
Substituting eq30 from the previous article in eq31,
where L’ = L – Eext ref elec + Eliq jn.
Since Eliq jn cannot be calculated or measured directly, the value of L’ must be determined by calibrating the pH meter with solutions of known pH.