Phase equilibria is a fundamental concept in thermodynamics that describes the balance between different phases of a substance or mixture at equilibrium. When a system reaches phase equilibrium, the rates of transition between phases are equal, resulting in no net change in the amount of each phase over time. This balance is governed by temperature, pressure and composition, and is represented using phase diagrams and equilibrium laws such as Raoult’s Law and the Clausius-Clapeyron equation.
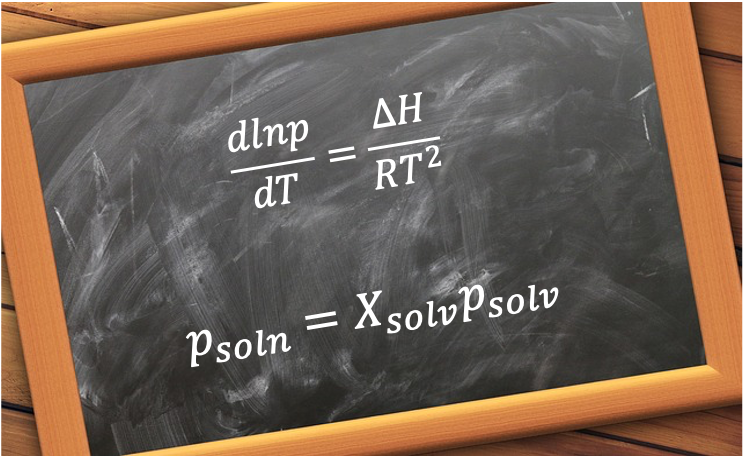
Understanding phase equilibria is crucial in both theoretical and applied sciences, as it explains phenomena like boiling, melting, sublimation and condensation. It also plays a vital role in fields such as material science, chemical engineering, and environmental science, where control over phase transitions is essential for the design and optimisation of various processes and products.