The non-crossing rule in quantum chemistry states that distinct eigenvalues of two eigenstates with the same symmetry cannot become equal as a function of an external perturbation parameter.
Consider a system with two orthogonal eigenstates and
of the unperturbed Hamiltonian
, with distinct eigenvalues of
and
, respectively. In the presence of a weak ligand field, the unperturbed Schrodinger equation
, becomes
where ,
is the perturbed Hamiltonian, and
is the eigenvalue corresponding to the perturbed eigenstate
, which is assumed to be a linear combination of the unperturbed eigenstates.
Substituting in eq281j gives
Multiplying eq281k from the left by and
in turn, and integrating over all space yields the following two equations:
where .
In matrix form, the pair of equations can be expressed as
where is the identity matrix.
Eq281l is a linear homogeneous equation, which has non-trivial solutions if
Expanding the determinant results in
whose roots are
The unperturbed Hamiltonian is invariant to all symmetry operations of a point group and transforms according to the fully symmetric representation of the group. In the context of a metal ion subject to a ligand field, the perturbed Hamiltonian is a summation of potentials between
metal valence electrons and
ligand valence electrons:
is also totally symmetric, as the distances between metal and ligand valence electrons
are invariant to all symmetry operations of a point group. If we assume that both
and
have a complete set of orthogonal eigenfunctions that are associated with real eigenvalues,
and
are Hermitian. This implies that
is also Hermitian. Using eq35, eq281n becomes
The term is positive and varies with the ligand field strength. If
and
have the same symmetry, then
is generally non-zero according to the vanishing integral principle. It follows that
and
are always distinct regardless of ligand field strength. This is known as the non-crossing rule.
On the other hand, if and
have different symmetry, then
is necessarily zero. Consequently, the eigenvalues of the two states may be the same at a certain ligand field strength where
.
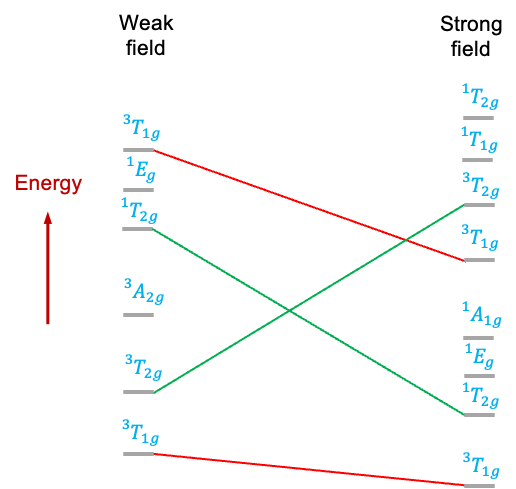
For example, the diagram below depicts some states of the configuration of a metal ion in an octahedral ligand field. The
states, which have the same symmetry, do not cross as the ligand field strength varies, while
and
, which have different symmetries, do.