What is the formula of the titration curve of a monoprotic strong acid versus a monoprotic strong base?
Consider a solution containing water, a strong acid (analyte) of concentration Ca and a strong base of concentration Cb with the following equilibria:
With reference to charge balance of the above equilibria, the sum of the number of moles of cations H+ and B+ must equal to that of anions A– and OH–. As the volume is of the solution is common to all ions,
Assume that the strong acid is fully ionised in water, at any point of the titration, the number of moles of A– must equal to the initial number of moles of HA. As the change in volume of the solution is common to all ions,
where Va and Vb are the volume of acid in the solution and the volume of base in the solution respectively.
Similarly, assuming that the strong base is fully ionised in water, at any point of the titration, the sum of the number of moles of B+ must equal to the number of moles of BOH added to the solution. As the change in volume of the solution is common to all ions,
Substitute eq2, eq3, Kw = [H+][OH–] and [H+] = 10-pH in eq1
Eq4 is the complete pH titration curve for a monoprotic strong acid versus monoprotic strong base system. We can input it in a mathematical software to generate a curve of pH against Vb. For example, if we titrate 10 cm3 of 0.200 M of HCl with 0.100 M of NaOH, we have the following:
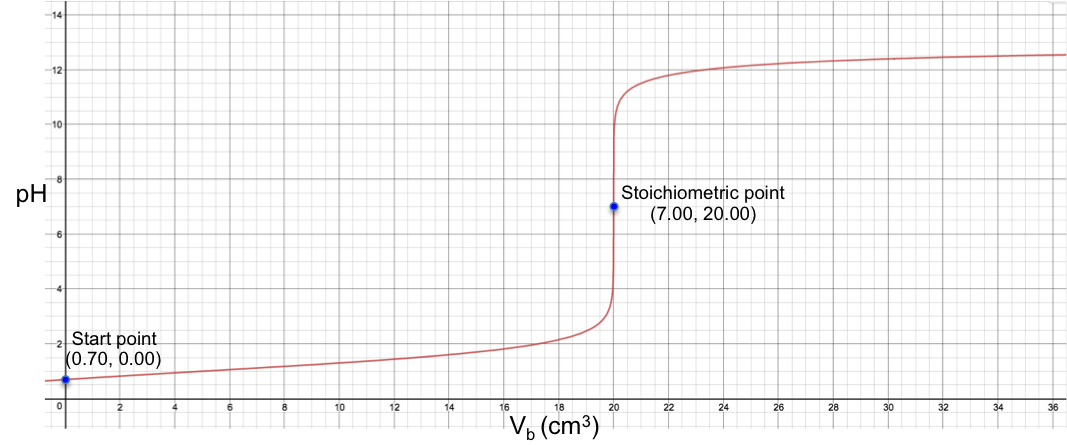
To understand the sharp change in pH near the stoichiometric point versus the change in Vb, we assume that one drop of base is about 0.05 cm3 and substitute 19.95 cm3, 20.00 cm3 and 20.05 cm3 into eq4. The respective pH just before and just after the stoichiometric point (SP) are:
|
One drop before SP |
SP |
One drop after SP |
|
|
Volume, cm3 |
19.95 | 20.00 |
20.05 |
|
pH |
3.78 | 7.00 |
10.22 |
The data shows that just two drops of base cause a drastic change of 6.44 in pH before and after the stoichiometric point. We can therefore use many different indicators that work within the range of the change in pH at the stoichiometric point to monitor the titration. Furthermore, we can derive the gradient equation for a monoprotic strong acid versus monoprotic strong base titration and investigate the inflexion point at pH 7 by rearranging eq4 as:
and finding by implicit differentiation to give:
At the stoichiometric point, CbVb – CaVa = 0 for a monoprotic acid-base reaction and the above equation becomes
Since [H+] = 10-7 M at the stoichiometric point for a strong acid versus strong base titration, the denominator of the above equation is a very small number and hence the gradient of the pH versus curve, or , is a very large value (computed to be 7238.2 cm-3), making at angle of 89.99o with the horizontal, i.e. a vertical line.

Question
How do we differentiate 10-2pH implicitly?
Answer
Let u = 10-2pH and use the chain rule where y = pH and x = Vb. The result is
.