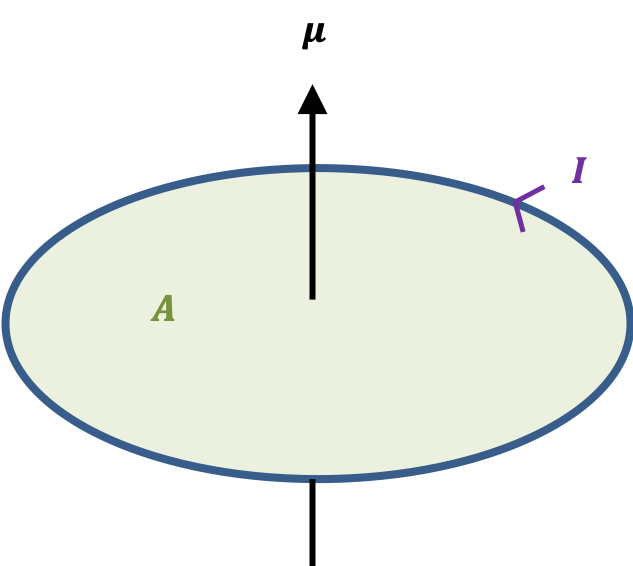
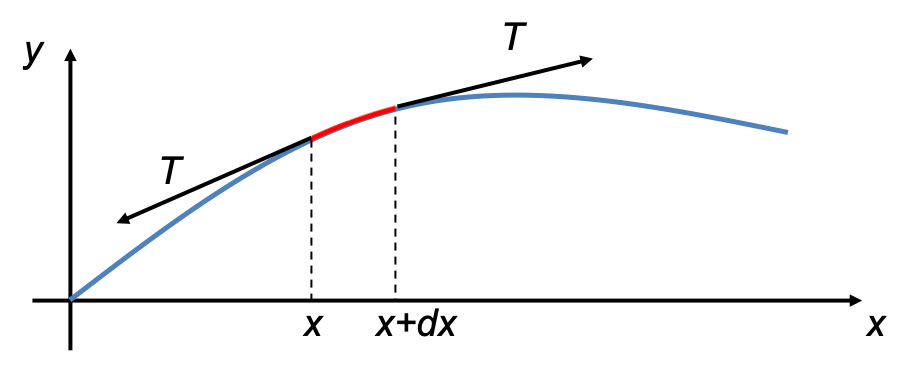
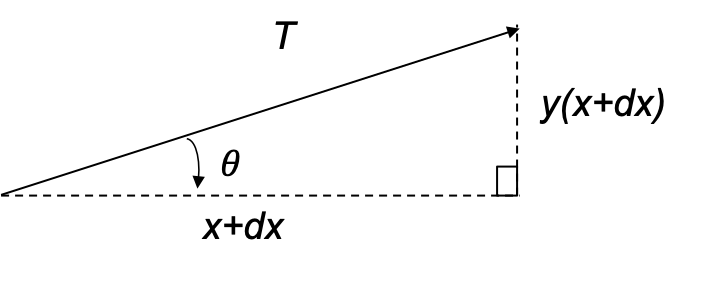
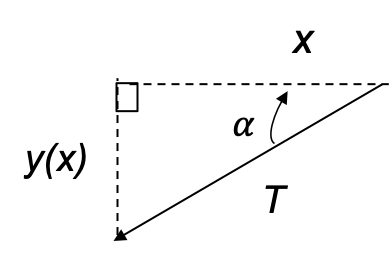
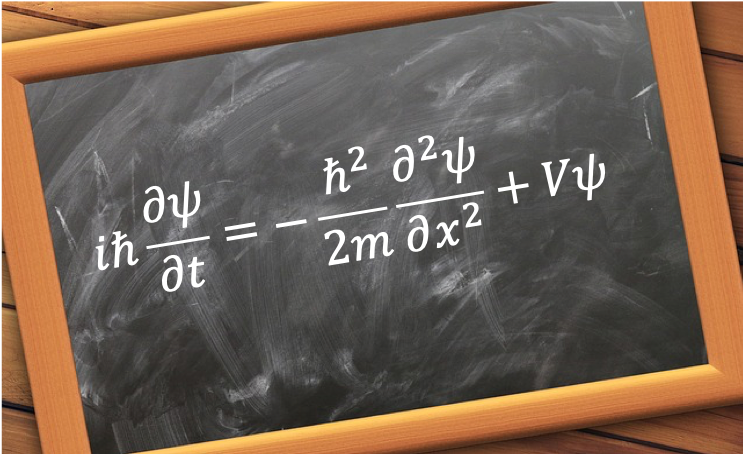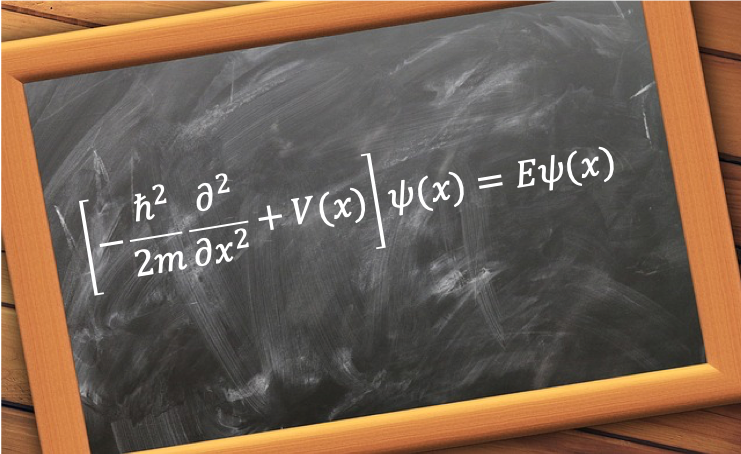The quantum orbital angular momentum operators in spherical coordinates are derived using the following diagram:
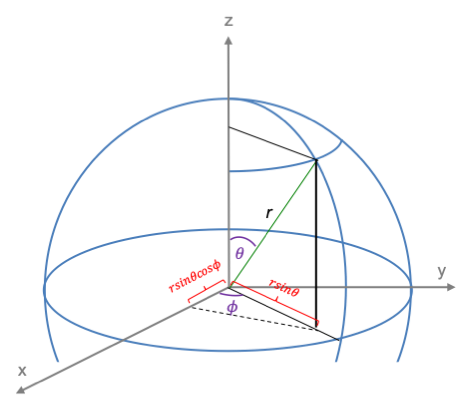
where
Therefore,
Similarly,
Furthermore, by differentiating implicitly with respect to
and separately with respect to
, and rearranging, we have

Question
Show that ,
and
.
Answer
To find expressions for and
, we let
for the first three equations of eq77, which gives us
,
and
. So,
Substituting these two expressions back into either the first or second equation of eq77, we have
Implicit differentiation of ,
and
with respect to
,
and
respectively gives
Since is independent of
Applying the multivariable chain rule to , we have:
Substitute i) eq78, eq81 and eq84 in eq87, ii) eq79, eq82 and eq85 in eq88 and iii) eq80, eq83 and eq86 in eq89, we have
respectively.
Substitute eq77, eq90, eq91 and eq92 in eq72, eq73 and eq74, we have
respectively.
Substitute eq93, eq94 and eq95 in eq75, we have, with some algebra
or equivalently
is the quantum orbital angular momentum operator and each of its eigenvalues is the square of the orbital angular momentum of an electron.

Question
Show that eq76 is in spherical coordinates.
Answer
Substitute eq93, eq94, eq95 and unit vectors in spherical coordinates ,
and
in eq76. we have, after some algebra, we have

Question
Show that .
Answer
Substituting eq97 in and using
,
,
and
, we have