A d-orbital, defined by the quantum number , is a region of space around the nucleus where an electron is most likely to be found. The letter ”d” is of spectroscopic origin, standing for ‘diffuse’.
One-electron d-orbital wavefunctions can be expressed using the total wavefunction of a hydrogenic atom:
where
, the radial wavefunction, is the radial component of
.
, the spherical harmonics, is the angular component of
.
are the associated Laguerre polynomials.
are the associated Legendre polynomials.
is the normalisation constant of the radial wavefunction.
is the normalisation constant of the spherical harmonics.
is the principal quantum number, where
is the orbital angular momentum quantum number (also known as azimuthal quantum number), where
and
.
is the magnetic quantum number, where
.
The principal quantum number, , is also called a shell. Since
when
, there are five d-orbitals that are characterised by the set of quantum numbers
of
,
,
,
and
in each shell for
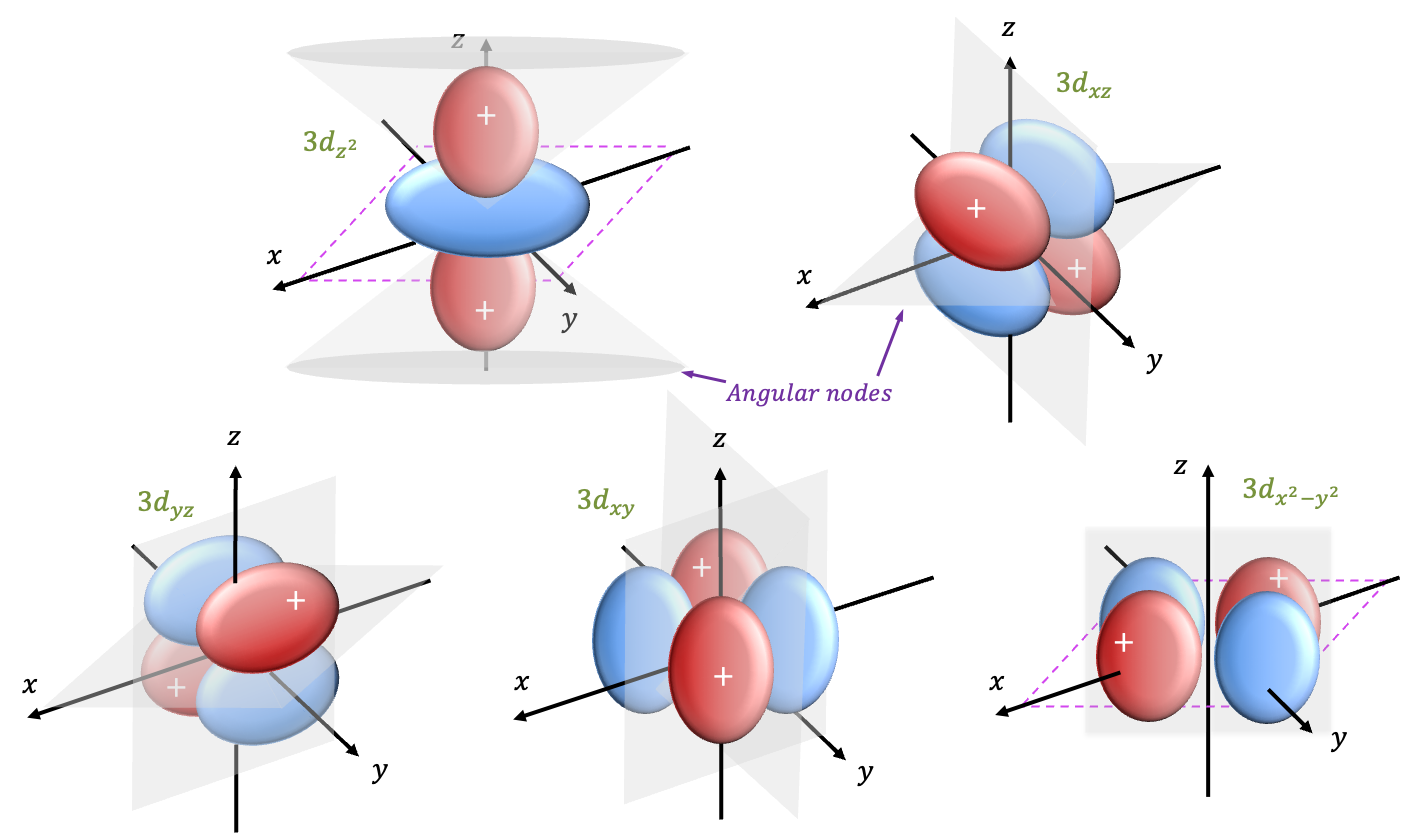
. Four of the five d-orbitals have cloverleaf shapes, while the fifth has a lobular structure along the
-axis with a doughnut-shaped region around the equatorial plane. Each of the five d-orbitals has two angular nodes. Consider the set
. Substituting these values into the explicit formula for
yields:
where .
Converting from spherical coordinates to Cartesian coordinates, where , gives
Eq478 is known as the wavefunction for the orbital. When
or
,
. Therefore, the wavefunction is zero at
in spherical coordinates. This implies that the angular nodes occur at two conical surfaces with their apices at the origin, extending at
along the
-axis.
For the sets and
, we have
and
, respectively. These two wavefunctions include the factor
, which may complicate calculations when they undergo symmetry operations. Therefore, we take linear combinations of these wavefunctions to form simpler wavefunctions. The first linear combination is
, which normalises to
, or equivalently in Cartesian coordinates:
where and
.
when
or
. This implies that
has two nodal planes:
and
.

Question
If and
are already normalised, why do we need to normalise a linear combination of them? How do we normalise a linear combination of
and
?
Answer
When forming a linear combination of normalised wavefunctions, the result is not necessarily normalised. Consider a general linear combination , where
and
are coefficients. The normalisation condition for
requires that
, where
represents the volume element in spherical coordinates. Expanding
and noting that
and
are orthonormal, we have
. If
, which is the case for the two linear combinations of
and
,
will not be normalised.
To normalise , we have
or
Since and
,
Using (see this article for proof) and setting
and
gives
.
The second normalised linear combination is , or equivalently,
where and
.
when
or
. This implies that
has two nodal planes:
and
.
For the remaining sets and
, we apply the same logic to give
and
, where
.
when
or
, which implies two diagonal nodal planes.
when
or
, which implies two nodes described by the planes
and
.