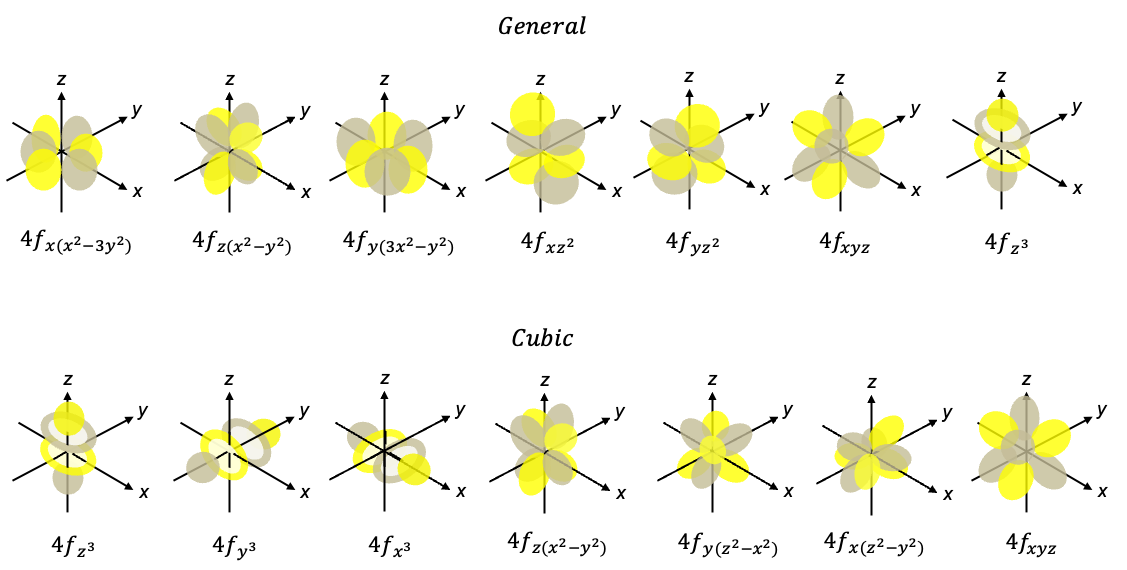
An f-orbital, defined by the quantum number , is a region of space around the nucleus where an electron is most likely to be found. The letter ”f” is of spectroscopic origin, standing for ‘fundamental’. f-block elements have either hexagonal close pack, cubic close pack or body centred cubic structures. For non-cubic symmetry systems, we describe them using a general set of orbitals, which are derived in the same way as the d-orbitals. For cubic symmetry systems, they are better described by another set of f-orbitals known as the cubic set, which are derived by taking a different set of linear combinations. Both sets are eigenfunctions of the Schrödinger equation.
One-electron f-orbital wavefunctions can be expressed using the total wavefunction of a hydrogenic atom:
where
, the radial wavefunction, is the radial component of
.
, the spherical harmonics, is the angular component of
.
are the associated Laguerre polynomials.
are the associated Legendre polynomials.
is the normalisation constant of the radial wavefunction.
is the normalisation constant of the spherical harmonics.
is the principal quantum number, where
is the orbital angular momentum quantum number (also known as azimuthal quantum number), where
and
.
is the magnetic quantum number, where
.
General set
The principal quantum number, , is also called a shell. Since
when
, there are seven f-orbitals that are characterised by the set of quantum numbers
of
,
,
,
,
,
and
in each shell for
. Each of the seven f-orbitals has three angular nodes. Consider the set
. Substituting these values into the explicit formula for
yields:
where .
Converting from spherical coordinates to Cartesian coordinates, where , gives
Eq482 is known as the orbital. When
or
or
,
.
represents the
–nodal plane, while
implies that the angular nodes occur at two conical surfaces with their apices at the origin, extending at
along the
-axis.
For the sets and
, we have
and
, respectively. These two wavefunctions include the factor
, which may complicate calculations when they undergo symmetry operations. Therefore, we take linear combinations of these wavefunctions to form simpler wavefunctions. The first linear combination is
, which normalises to
, or equivalently in Cartesian coordinates:
where ,
and
.
One angular node occurs at or when
, which represents the
-nodal-plane. The second and third angular nodes correspond to
. These nodes are described by two conical surfaces with their apices at the origin, extending at
along the
-axis.
The second normalised linear combination is , or equivalently,
where .
One angular node occurs at , which represents the
-nodal plane. The second and third angular nodes correspond to
. These nodes are described by two conical surfaces with their apices at the origin, extending at
along the
-axis.

Question
If and
are already normalised, why do we need to normalise a linear combination of them? How do we normalise a linear combination of
and
?
Answer
When forming a linear combination of normalised wavefunctions, the result is not necessarily normalised. Consider a general linear combination , where
and
are coefficients. The normalisation condition for
requires that
, where
represents the volume element in spherical coordinates. Expanding
and noting that
and
are orthonormal, we have
. If
, which is the case for the two linear combinations of
and
,
will not be normalised.
To normalise , we have
or
Using (see this article for proof),
and setting
and
gives
.
For the remaining sets ,
,
and
, we apply the same logic to give:
| Wavefunction | Angular nodes | |
|
|
||
|
|
||
|
|
||
|
|
Cubic set
Out of the seven cubic f-orbitals, three of them, ,
and
are the same as the general set of f-orbitals. The other four derived by taking different linear combinations of general set of f -orbitals. They are:
| Linear combination | Normalised wavefunction | Angular nodes | |
|
Conical surfaces with apices at the origin, extending |
|||
|
Conical surfaces with apices at the origin, extending |
|||
|
|
|||
|
|

Question
-
- How do we know which set of linear combinations result in the cubic set of wavefunctions?
- How do we determine the angular nodes of
?
Answer
-
- The linear combinations that give the cubic set of wavefunctions must transform according to the
point group.
- The angular nodes occur when
, which implies that
or
. Obviously,
corresponds to the
-nodal-plane.
, which states that
increases as
increases in spherical coordinates, represents two conical surfaces with their apices at the origin. Substituting
gives
. When
(along the
-axis), the angles that the conical surfaces make with the
-axis are
.
- The linear combinations that give the cubic set of wavefunctions must transform according to the