Group theory allows us to determine the symmetries of a molecule, enabling an efficient way to obtain relevant information in infra-red (IR) spectroscopy.
The objective in this article is to use group theory to work out the symmetries of the normal modes of a molecule and then ascertain whether they are IR-active. As an example, let’s consider  , which belongs to the
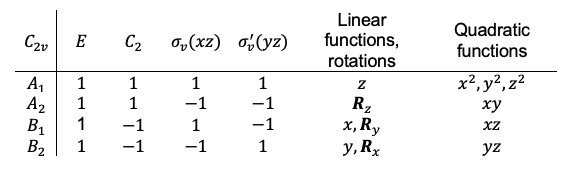
, which belongs to the  point group. Here’s the corresponding character table:
point group. Here’s the corresponding character table:
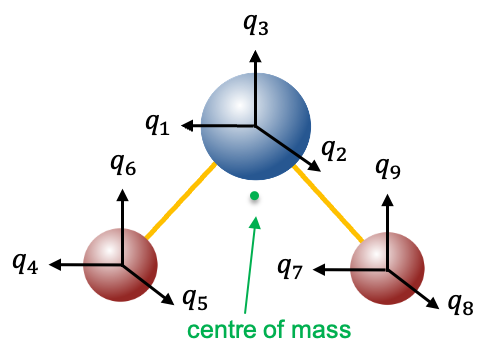
Instead of using Cartesian displacement vectors to form a basis for generating representations of the  point group, as we did in the previous article, we shall use mass-weighted Cartesian coordinate unit vectors
point group, as we did in the previous article, we shall use mass-weighted Cartesian coordinate unit vectors 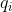 to form a basis (see diagram above). Since a symmetry operation of
to form a basis (see diagram above). Since a symmetry operation of  transforms
transforms  into an indistinguishable copy of itself, it transforms each of the elements of the basis set into a linear combination of elements of the set (see eq55 to eq61). Therefore,
into an indistinguishable copy of itself, it transforms each of the elements of the basis set into a linear combination of elements of the set (see eq55 to eq61). Therefore, 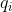 centred on every atom of
centred on every atom of  generate a representation
generate a representation  of the
of the  point group. To find the matrices of the representation, we arrange the vectors into a row vector and apply the symmetry operations of the
point group. To find the matrices of the representation, we arrange the vectors into a row vector and apply the symmetry operations of the  point group on it to obtain the transformed vector. The matrices of the representation are then constructed by inspection. For example,
point group on it to obtain the transformed vector. The matrices of the representation are then constructed by inspection. For example,
\xrightarrow{C_2}\(-q_1,-q_2,q_3,-q_7,-q_8,q_9,-q_4,-q_5,q_6\))

The traces of the matrices are

Using eq27a and the character table of the  point group, we have
point group, we have
which implies that the decomposition of the reducible representation is  .
.
Applying the projection operator on each element of the  basis set for all irreducible representations, we have
basis set for all irreducible representations, we have
Each equation in the above table is a basis of the irreducible representation it belongs to. Since any linear combination of bases that transform according to a one-dimensional irreducible representation is also a basis of that irreducible representation, we can generate symmetry-adapted linear combinations (SALC) from the above basis vectors that belong to their respective irreducible representations.
We have shown in an earlier article that
-
- the number of orthogonal or orthonormal basis functions of a representation corresponds to the dimension of the representation.
- if
 is a basis of a representation
is a basis of a representation 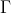 of a group, then any linear combination of
of a group, then any linear combination of  is a basis of a representation
is a basis of a representation  that is equivalent to
that is equivalent to 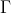 .
.
This implies that we can always select a set of nine orthonormal SALC for a block diagonal representation that is equivalent to  . Since none of the nine SALCs can be expressed as a linear combination of the others, each SALC, known as normal coordinates
. Since none of the nine SALCs can be expressed as a linear combination of the others, each SALC, known as normal coordinates 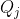 , must describe one of nine independent motions of the molecule.
, must describe one of nine independent motions of the molecule.
A quick method to assign the irreducible representations from the decomposition of  to the nine degrees of freedom of
to the nine degrees of freedom of 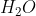 is to refer to the character table, where the basis functions
is to refer to the character table, where the basis functions 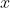 ,
, 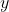 and
and  represent the three independent translational motion and
represent the three independent translational motion and  ,
,  and
and  represent the three independent rotational motion. Deducting the irreducible representations associated with these six basis functions from the direct sum of
represent the three independent rotational motion. Deducting the irreducible representations associated with these six basis functions from the direct sum of  , we are left with
, we are left with 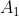 ,
, 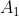 and
and 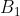 . These remaining three irreducible representations correspond to the vibrational degrees of freedom.
. These remaining three irreducible representations correspond to the vibrational degrees of freedom.
To verify whether the three normal modes are IR-active, we refer to the Schrodinger equation for vibration motion (see eq93):

where the separation of variables technique allows us to approximate the total vibrational wavefunction as 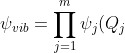) and hence,
and hence,  .
.
Each 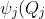) describes a normal mode of the molecule and has the formula (see eq94):
describes a normal mode of the molecule and has the formula (see eq94):
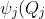=N_jH_j\biggr\(\sqrt{\frac{\omega_j}{\hbar}}Q_j\biggr\)e^{-\frac{\omega_jQ^2_j}{2\hbar}})
where  is the normalisation constant for the Hermite polynomials
is the normalisation constant for the Hermite polynomials  .
.
The total vibrational energy (see eq96) is
\hbar\omega_j)
A vibrational state of a polyatomic molecule  is characterised by
is characterised by 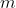 quantum numbers. Hence, the vibrational ground state of
quantum numbers. Hence, the vibrational ground state of 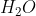 , where
, where  , is
, is  or
or
e^{-\frac{\omega_jQ_j^2}{2\hbar}}=N\prod_{j=1}^3e^{-\frac{\omega_jQ_j^2}{2\hbar}})
Many IR-spectroscopy experiments are conducted at room temperature, where most molecules are in their vibrational ground state. According to the time-dependent perturbation theory, the transition probability between orthogonal vibrational states within a given electronic state of a molecule is proportional to

where  and
and  are the initial and final states respectively and
are the initial and final states respectively and  is the operator for the molecule’s electric dipole moment.
is the operator for the molecule’s electric dipole moment.
In other words, no transition between states occurs when  . Since the objective is to ascertain the IR activity of each of the three normal modes of
. Since the objective is to ascertain the IR activity of each of the three normal modes of 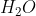 , it is suffice to study the fundamental transitions, where
, it is suffice to study the fundamental transitions, where  and
and  or
or  or
or  .
.
The next step involves determining which irreducible representation of the  point group
point group  ,
,  ,
,  ,
, 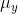 and
and  belong to. Since the components
belong to. Since the components  ,
, 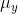 and
and  represent the electric dipole moment along the
represent the electric dipole moment along the 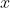 ,
, 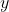 and
and  directions respectively, they transform in the same way as the basis functions
directions respectively, they transform in the same way as the basis functions 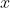 ,
, 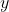 and
and  respectively (see character table above).
respectively (see character table above).

Question
Show that  , where
, where  represents the symmetry operations of a point group and
represents the symmetry operations of a point group and  .
.
Answer
In general, if =f(x)) , then the function
, then the function 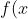) is invariant under the symmetry operation
is invariant under the symmetry operation  . Every value of
. Every value of 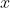 is mapped into
is mapped into 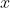 by
by  and we can say that
and we can say that  if
if =f(x)) . The converse is also true, i.e. if the variable
. The converse is also true, i.e. if the variable 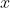 of the function
of the function 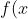) transforms according to
transforms according to  , then
, then =f(x)) . From this article, the potential term of the vibrational Hamiltonian for
. From this article, the potential term of the vibrational Hamiltonian for 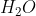 is
is =U_e+\frac{1}{2}\sum_{j=1}^{3N-6}\lambda_jQ^2_j}) . Since the function
. Since the function ) is invariant under any symmetry operation,
is invariant under any symmetry operation, =U_e+\frac{1}{2}\sum_{j=1}^{3N-6}\lambda_jQ^2_j=U_e+\frac{1}{2}\sum_{j=1}^{3N-6}\lambda_j(R_{\alpha}Q_j)^2}) or simply
or simply
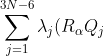^2=\sum_{j=1}^{3N-6}\lambda_jQ_j^2\;\;\;\;\;\;\;\;105)
If each 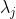 is distinct, then
is distinct, then ^2=Q_j^2) or
or

If 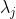 is degenerate, eq55 states that
is degenerate, eq55 states that  and eq105 becomes
and eq105 becomes
^2+\lambda_1(a_{12}Q_1+a_{22}Q_2)^2+\lambda_3(R_{\alpha}Q_3)^2+\cdots=\\\lambda_1Q_1^2+\lambda_1Q^2_2+\lambda_3Q_3^2+\cdots\;\;\;\;\;\;\;\;107)
where we have assumed that 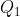 and
and 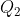 are degenerate.
are degenerate.
As described in this article, 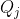 forms a set of orthonormal eigenvectors of the vibrational Hamiltonian. So,
forms a set of orthonormal eigenvectors of the vibrational Hamiltonian. So,  and
and  are orthonormal vectors. The matrix formed by using these vectors as its columns is an orthogonal matrix
are orthonormal vectors. The matrix formed by using these vectors as its columns is an orthogonal matrix 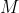 , which is defined as
, which is defined as  . This is because the dot product of different columns will be zero, and the dot product of a column with itself will be 1. Since
. This is because the dot product of different columns will be zero, and the dot product of a column with itself will be 1. Since  if
if 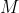 is an orthogonal matrix (see property 11 of this link for proof),
is an orthogonal matrix (see property 11 of this link for proof),  or
or

which implies that  and
and  .
.
The first two terms on LHS of eq107 then becomes  . This implies that
. This implies that ^2) must also be equal to
must also be equal to  in eq105 if
in eq105 if 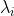 is degenerate. Therefore,
is degenerate. Therefore,  regardless of whether
regardless of whether 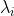 is non-degenerate or degenerate.
is non-degenerate or degenerate.
As mentioned in the above Q&A, if the variable 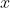 of the function
of the function 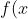) transforms according to
transforms according to  , then
, then =f(x)) . It follows that if the variable
. It follows that if the variable 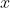 of the function
of the function 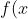) transforms according to
transforms according to  , then
, then =f(-x)) , in which case
, in which case  is the reflection operator about the vertical axis of the graph of
is the reflection operator about the vertical axis of the graph of 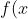) against
against 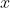 . Therefore, the ground state vibrational wave function
. Therefore, the ground state vibrational wave function  of
of 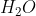 transforms according to the totally symmetric irreducible representation
transforms according to the totally symmetric irreducible representation 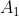 of the
of the  point group because
point group because
=\psi_0(\pm Q_j)=N\prod_{j=1}^3e^{-\frac{\omega_j(\pm Q_j)^2}{2\hbar}}=\psi_0)
Next, let’s analyse the symmetries of  ,
,  and
and  . These three wave functions (see this article) have the same form of:
. These three wave functions (see this article) have the same form of:

Eq108 is a product of two functions  and
and  . Since
. Since  is totally symmetric, the theory of direct product representation states that
is totally symmetric, the theory of direct product representation states that  must transform according to the irreducible representation of the
must transform according to the irreducible representation of the  point group that
point group that  belongs to.
belongs to.
With reference to the  character table above, the theory of direct product representation again finds that the functions
character table above, the theory of direct product representation again finds that the functions  ,
,  and
and  in eq104 transform according to the irreducible representations of
in eq104 transform according to the irreducible representations of 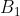 ,
,  and
and 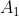 respectively. Therefore, the theory of vanishing integrals states that
respectively. Therefore, the theory of vanishing integrals states that
|
 |
 |
 |
Zero |
Not zero |
 |
Zero |
Zero |
 |
Not zero |
Zero |
Since  in eq104 for each of the three normal modes, all three normal modes of
in eq104 for each of the three normal modes, all three normal modes of 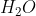 are IR-active.
are IR-active.

is an even number,
is an odd number, we too end up with eq29. Therefore,
in eq29 represents any number. Taking the derivative of eq29 again,
in eq31 with
gives

, show that eq32 can be used to generate the Hermite polynomials.
in eq32, we have
. Substituting
in eq32, we have
. Repeating the logic, we can generate the rest of the polynomials.

and eq46, show that
.
and integrating over all space,
, where
are the normal modes, into eq32a gives:
is given by eq94.
,