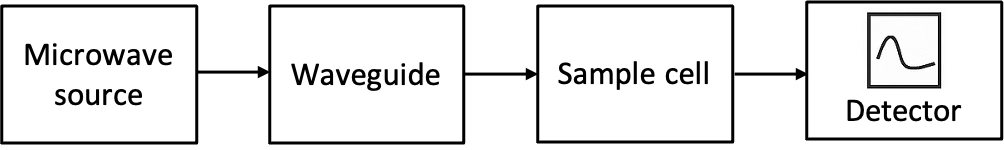
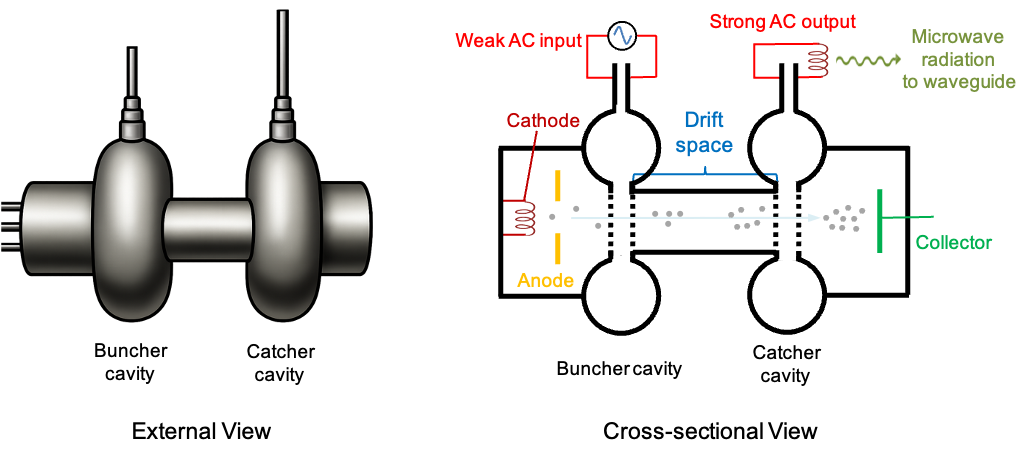
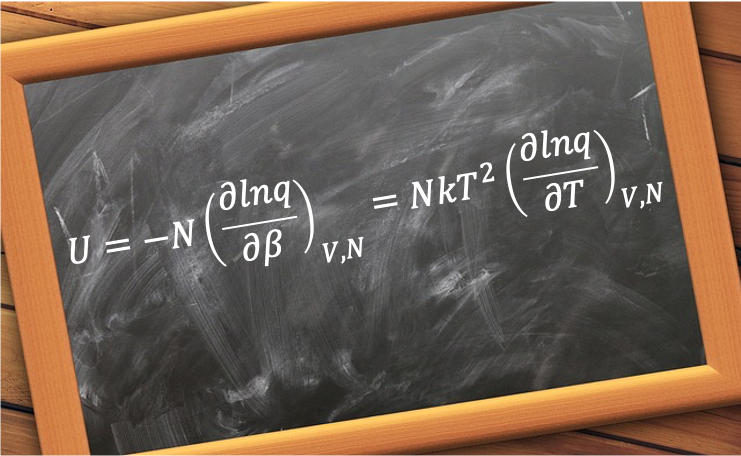Microwave spectra represent the electromagnetic radiation absorbed or emitted by molecules when they undergo transitions between discrete rotational energy levels. These spectra are a key component in the study of molecular structure and dynamics. They provide valuable insights about molecular properties, such as bond lengths and moments of inertia.
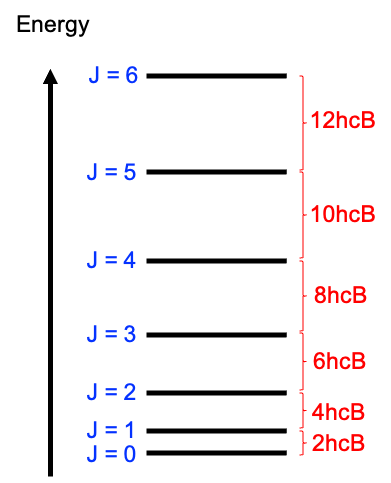
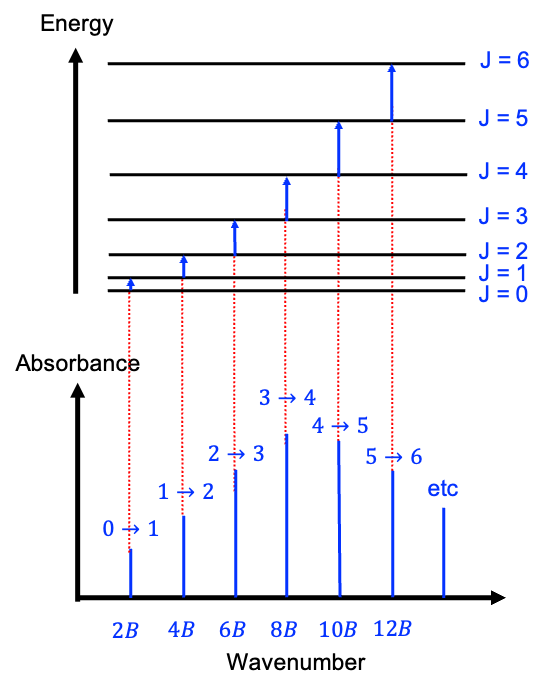
An ideal, pure absorption rotational spectrum of a rigid linear molecule, generated in the microwave frequency range of 3 to 600 GHz (1 to 200 cm-1), typically exhibits a series of absorption lines that correspond to the rotational transitions between discrete rotational energy levels (see diagram below). In the absence of fine or hyperfine splitting, these transitions follow the selection rule  , meaning that a molecule can only transition from one rotational level to the next higher level.
, meaning that a molecule can only transition from one rotational level to the next higher level.

Question
What is the difference between an absorption spectrum and an emission spectrum?
Answer
An external radiation is required to produce an absorption spectrum. The detector measures the amount of energy absorbed by the sample at each frequency, corresponding to transitions with  .
.
In an emission spectrum, the sample itself acts as the source. It must first be excited to higher-energy rotational states, for example by heating or applying an electrical discharge. The detector then measures the amount of energy emitted by the sample, corresponding to transitions with  .
.
Both types of spectra can be obtained using the same spectrometer, with different settings. Suppose the spectrometer is configured to record the absorption spectrum of a sample. If two rotational states are populated under the experimental conditions, the sample can both absorb and emit radiation at the same frequency, since both processes involve transitions with  between the same pair of quantised energy levels. However, the conditions for measuring an absorption spectrum are typically set to ensure that net absorption occurs.
between the same pair of quantised energy levels. However, the conditions for measuring an absorption spectrum are typically set to ensure that net absorption occurs.
The rotational energy levels are given by eq44 or eq45 and the frequencies of the transitions are directly related to the rotational constant 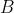 , and as such, the spacing between the spectral lines provides information about the moment of inertia and the molecular structure. Using the definition
, and as such, the spacing between the spectral lines provides information about the moment of inertia and the molecular structure. Using the definition ) , we have
, we have
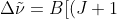(J+2)-J(J+1)]=2B(J+1)\;\;\;\;\;\;\;\;80)
Therefore, the first peak, obtained by substituting 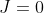 into eq80, lies at the point
into eq80, lies at the point  along the horizontal axis. Similarly, the second and third peaks are at
along the horizontal axis. Similarly, the second and third peaks are at  and
and  respectively. In other words, the peaks in an ideal rotational spectrum (without centrifugal distortion) of a rigid linear rotor are equally spaced at
respectively. In other words, the peaks in an ideal rotational spectrum (without centrifugal distortion) of a rigid linear rotor are equally spaced at  .
.
The intensity of each transition  depends on the population of the initial level
depends on the population of the initial level 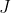 , which is governed by the Boltzmann distribution, with higher-
, which is governed by the Boltzmann distribution, with higher-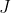 states being less populated at lower temperatures:
states being less populated at lower temperatures:

where
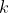 is the Boltzmann constant.
is the Boltzmann constant.
 is the number of particles in the energy state
is the number of particles in the energy state  .
.
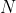 is the total number of particles in the system.
is the total number of particles in the system.
Since  represents the fraction of particles in the state
represents the fraction of particles in the state  , the intensity of a spectral line is proportional to
, the intensity of a spectral line is proportional to  , where the value
, where the value  corresponds to a specific energy level. However, there are
corresponds to a specific energy level. However, there are ) degenerate states associated with each value of
degenerate states associated with each value of 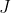 in a rigid linear molecule. This degeneracy increases the statistical weight of higher
in a rigid linear molecule. This degeneracy increases the statistical weight of higher 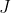 levels, redistributing the population towards more degenerate states, which become thermally accessible at typical laboratory temperatures. In other words, at a given temperature, more particles will occupy a higher
levels, redistributing the population towards more degenerate states, which become thermally accessible at typical laboratory temperatures. In other words, at a given temperature, more particles will occupy a higher 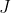 state with greater degeneracy at equilibrium than they would if the state were non-degenerate. Therefore, when a sample is exposed to an external microwave field, the intensity
state with greater degeneracy at equilibrium than they would if the state were non-degenerate. Therefore, when a sample is exposed to an external microwave field, the intensity 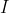 of each allowed transition in the pure rotational spectrum is proportional to the population of the initial state, which is the product of
of each allowed transition in the pure rotational spectrum is proportional to the population of the initial state, which is the product of  and
and ) . This gives:
. This gives:
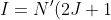e^{-\frac{hcBJ(J+1)}{kT}}\;\;\;\;\;\;\;\;81)
where  is a proportionality constant and
is a proportionality constant and  is given by eq45.
is given by eq45.
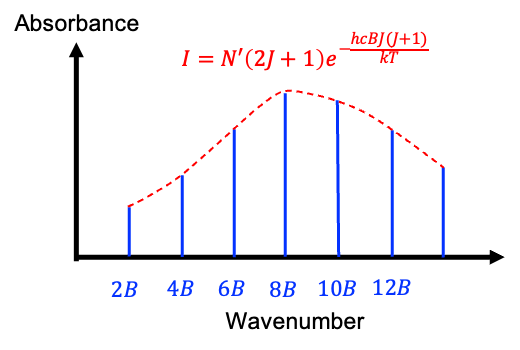
The factor ) in eq81 increases linearly with
in eq81 increases linearly with 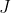 , while the exponential term decreases exponentially. As a result, the graph of
, while the exponential term decreases exponentially. As a result, the graph of 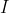 versus
versus 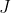 forms a skewed bell curve (see diagram above), with a maximum found by treating
forms a skewed bell curve (see diagram above), with a maximum found by treating 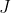 as a continuous variable and taking the derivative of
as a continuous variable and taking the derivative of 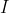 with respect to
with respect to 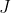 :
:
}{kT}}\biggr\[2-(2J+1)^2\frac{hcB}{kT}\biggr\])
Setting  , and noting that
, and noting that }{kT}}\neq 0) for any finite
for any finite 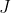 , gives
, gives

Since 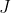 must be an integer, the maximum intensity occurs at the value of
must be an integer, the maximum intensity occurs at the value of 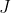 closest to the result of eq82.
closest to the result of eq82.

Question
Is the intensity of each transition dependent on the electric dipole moment?
Answer
Although the transition probability between adjacent rotational states is proportional to _{l',m'_l}\vert\boldsymbol{\mathit{\hat{\mu}}}\vert\psi(\theta,\phi)_{l,m_l}\rangle\vert^2) , which depends on the magnitude of the electric dipole moment, the sample molecules in the waveguide of a typical microwave spectrometer are randomly oriented. As a result, the effect transition dipole moment does not vary significantly from one
, which depends on the magnitude of the electric dipole moment, the sample molecules in the waveguide of a typical microwave spectrometer are randomly oriented. As a result, the effect transition dipole moment does not vary significantly from one  transition to the next for a given molecule. Therefore, while the absolute intensity depends on the dipole moment, it is often treated as a constant factor when comparing transitions within the same molecule.
transition to the next for a given molecule. Therefore, while the absolute intensity depends on the dipole moment, it is often treated as a constant factor when comparing transitions within the same molecule.
For a symmetric rotor, +K^2(\tilde{A}-\tilde{B})) and
and  is also given by eq80. However, unlike a linear rotor, each allowed transition receives contributions from multiple
is also given by eq80. However, unlike a linear rotor, each allowed transition receives contributions from multiple 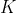 states. These overlapping contributions increase the intensity of the lines but do not change their positions for an ideal spectrum. Consequently, the overall intensity envelope of the spectrum still resembles a skewed bell curve, with a maximum at the value of
states. These overlapping contributions increase the intensity of the lines but do not change their positions for an ideal spectrum. Consequently, the overall intensity envelope of the spectrum still resembles a skewed bell curve, with a maximum at the value of 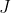 corresponding to the highest population.
corresponding to the highest population.
In practice, the peaks in a real rotational spectrum are not discrete vertical lines but have finite width and shape, appearing as broadened curves. This broadening arises, under typical laboratory conditions, from several effects:
-
- Doppler broadening, due to the thermal motion of molecules, causes a spread in observed frequencies as molecules move towards or away from the detector.
- Instrumental broadening, resulting from the finite resolution of the spectrometer itself.
- Lifetime broadening, which is based on the energy-time uncertainty relation.
Beyond broadening, other phenomena affect the detailed structure and spacing of the spectral lines:
-
- Centrifugal distortion causes deviations from the ideal rigid rotor model. As rotational speed increases at higher
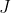 levels, the molecular bond stretches slightly, increasing the moment of inertia and decreasing the rotational constant
levels, the molecular bond stretches slightly, increasing the moment of inertia and decreasing the rotational constant 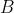 . This leads to uneven spacing between lines, especially at higher
. This leads to uneven spacing between lines, especially at higher 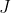 , and is accounted for using a correction term.
, and is accounted for using a correction term.
-
- Isotopic substitution alters the moment of inertia due to changes in atomic mass, thereby changing the rotational constant
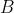 . Different isotopologues of the same molecule produce distinct sets of rotational lines, each with slightly different spacings. If multiple isotopes are present in the sample, the resulting spectrum may show clusters or duplications of lines, corresponding to each isotopologue.
. Different isotopologues of the same molecule produce distinct sets of rotational lines, each with slightly different spacings. If multiple isotopes are present in the sample, the resulting spectrum may show clusters or duplications of lines, corresponding to each isotopologue.
Together, these effects lead to a rotational spectrum that is richer and more complex than the idealised, evenly spaced series of lines predicted by the rigid rotor model.

Question
Do large molecules, such as polymers, enzymes and DNA, undergo rotational transitions when radiated by microwaves?
Answer
Yes, large molecules can undergo rotational transitions when exposed to microwave radiation. However, such molecules are flexible and do not behave like rigid rotors, which invalidates some of the assumptions used in microwave rotational spectroscopy. If we assume that ) still applies, the rotational energy levels will be very closely spaced because the moment of inertia
still applies, the rotational energy levels will be very closely spaced because the moment of inertia  is large, and
is large, and  becomes very small. As a result, individual rotational transitions for large molecules often overlap, leading to broad, unresolved bands or a complete loss of identifiable peaks.
becomes very small. As a result, individual rotational transitions for large molecules often overlap, leading to broad, unresolved bands or a complete loss of identifiable peaks.
Furthermore, instead of undergoing uniform rotation, different parts of these complex molecules can move independently in response to the microwave field, including side-chain rotations and wobbling. These movements cause the molecules to collide with neighbouring molecules, converting the absorbed microwave energy into kinetic energy and heat. This general heating effect may denature the large molecules, leading to further changes in their structure and, consequently, variations in the observed spectra.
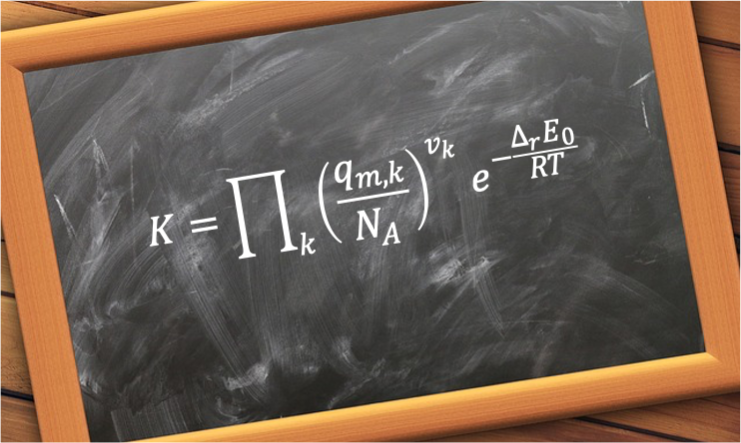
is the stoichiometric coefficient of the species
in the reversible reaction. Note that the stoichiometric coefficients for reactants are negative by convention, while those for products are positive.
.

.
is derived using
(see this article), we need to show that the statistical thermodynamics expression for the chemical potential of an ideal gas can be transformed into
. Substituting eq257 into eq330 yields:
.
into eq385 gives:
. Then,
is the activity of an ideal gas.