The analytical solution of the energy equation of He involves finding analytical expressions for all terms in eq8 using trial one-electron wavefunctions. As per the numerical method, guess values are used for the variables in all trial one-electron wavefunctions except one, e.g. ) . Eq8 is then minimised to obtain the solutions for
. Eq8 is then minimised to obtain the solutions for 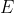 and
and ) . The process is repeated until
. The process is repeated until 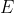 and all
and all ) become invariant. For He, eq8 is
become invariant. For He, eq8 is
)
where \left(-\frac{1}{2}\nabla_i^2-\frac{Z}{r_i}\right)\phi_i(\mathbf r_i)d\mathbf r_i) and
and  .
.
Our computation employs the following assumptions:
- We base our iteration on the unrestricted case, where electrons
 and
and  are distinctively expressed by
are distinctively expressed by  and
and  respectively.
respectively.
- All terms on the RHS of eq48 are determined using Slater-type orbitals, where

Substituting  in
in 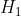 , using the identity
, using the identity  for
for  and integrating by parts for
and integrating by parts for  , we have
, we have
)
Similarly, substituting  in
in 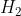 , we have
, we have
)
Substituting  ,
,  and eq47 in
and eq47 in  and multiplying the resultant equation by
and multiplying the resultant equation by ]^*Y_0^0(\theta_2,\phi_2)=1) , where
, where =\frac{1}{\sqrt{4\pi}}) is the spherical harmonics for
is the spherical harmonics for 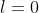 , we have
, we have
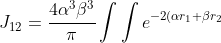}\sum_{n=0}^{\infty}\sum_{m=-n}^n\frac{4\pi}{2n+1}\frac{r_<^n}{r_>^{n+1}})
[Y_n^{\vert m\vert}(\theta_2,\phi_2)]^*[Y_0^0(\theta_1,\phi_1)]^*Y_0^0(\theta_2,\phi_2)d\boldsymbol{\mathit{r}}_1d\boldsymbol{\mathit{r}}_2)
Due to the orthogonality of the spherical harmonics, the only integral that survives upon expanding the summation is when  and
and 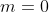 . Since,
. Since,  , the above equation becomes:
, the above equation becomes:

We proceed by integrating with respect to 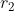 first. Since
first. Since 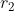 ranges from 0 to
ranges from 0 to 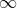 , we can split the integral into two parts, one from 0 to
, we can split the integral into two parts, one from 0 to 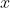 and the other from
and the other from 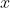 to
to 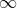 . Supposing
. Supposing  , the above equation becomes:
, the above equation becomes:
dr_1)
As 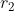 increases from 0 and approaches
increases from 0 and approaches 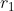 , it must be less than
, it must be less than 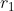 and so
and so  . Similarly, as
. Similarly, as 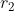 increases from
increases from 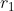 to
to 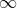 , it must be greater than
, it must be greater than 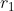 and so
and so  . Therefore,
. Therefore,

dr_1\;\;\;\;\;\;\;\;(52))
Using the identity ^i}{i!}\right]) for the first integral within parentheses, integrating by parts for the second integral within parentheses, and employing the identity
for the first integral within parentheses, integrating by parts for the second integral within parentheses, and employing the identity  for the integral with respect to
for the integral with respect to 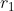 yields
yields
}{(\alpha+\beta)^3}\;\;\;\;\;\;\;\;55)
Substituting eq50, eq51 and eq55 in eq48 gives
}{(\alpha+\beta)^3}\;\;\;\;\;\;\;\;56)
Differentiating eq56 with respect to 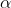 and
and 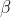 results in
results in
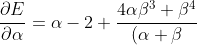^4}\;\;\;\;\;\;\;\;57)
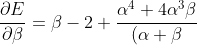^4}\;\;\;\;\;\;\;\;58)
Eq57 and eq58 are used in an iterative algorithm to find 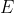 ,
,  and
and  . The procedure is as follows:
. The procedure is as follows:
- Substitute an initial guess value of
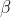 , e.g.,
, e.g.,  , in eq57 and solve for
, in eq57 and solve for 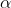 by setting eq57 equal to zero. We then substitute the solution of
by setting eq57 equal to zero. We then substitute the solution of 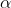 and the initial guess value of
and the initial guess value of 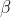 in eq56 to find
in eq56 to find 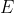 .
.
- To obtain an improved estimate of
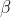 , we substitute the solution of
, we substitute the solution of 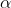 from the previous step in eq58, and solve for
from the previous step in eq58, and solve for 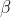 by setting eq58 equal to zero. We then substitute the solution of
by setting eq58 equal to zero. We then substitute the solution of 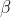 and the value of
and the value of 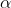 found in the previous step in eq56 to find a better estimate of
found in the previous step in eq56 to find a better estimate of 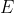 .
.
- Steps 1 and 2, which form an iteration set, are repeated until the values of
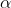 and
and 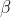 are invariant up to six decimal points.
are invariant up to six decimal points.
Alternatively, we can set up an iterative table in Excel as follows:

Cell C2 is the initial guess value of 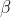 . The formula for D2:D7 is eq56, i.e. =(B2^2)/2-2*B2+(C2^2)/2-2*C2+(B2*C2*((B2^2)+3*B2*C2+(C2^2)))/((B2+C2)^3). The formulae for B3, C4, B5, C6 and B7 are =B2, =C3, =B4, =C5 and =B6, respectively. To compute the first iteration set, we employ the Excel Solver application to minimise D2 with respect to B2, with the following settings:
. The formula for D2:D7 is eq56, i.e. =(B2^2)/2-2*B2+(C2^2)/2-2*C2+(B2*C2*((B2^2)+3*B2*C2+(C2^2)))/((B2+C2)^3). The formulae for B3, C4, B5, C6 and B7 are =B2, =C3, =B4, =C5 and =B6, respectively. To compute the first iteration set, we employ the Excel Solver application to minimise D2 with respect to B2, with the following settings:
Set objective: D2
To: Min
By changing variable cells: B2
Selecting a solving method: GRG Nonlinear
We then solve for D3 by changing the ‘Set objective’ field and ‘By changing variable cells’ field to D3 and C3 respectively. This procedure is repeated for subsequent iteration sets until the values of 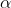 and
and 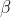 are invariant up to six decimal points.
are invariant up to six decimal points.
The results are as follows:

The final theoretical value of  has a deviation of about 1.93% versus the experiment data of the ground state of helium.
has a deviation of about 1.93% versus the experiment data of the ground state of helium.

, where
and
is given by eq66.

and
is Hermitian.
.
, we have
. Using eq65, we have
, i.e. the product of
and
is Hermitian. This implies that
, where
is also Hermitian.
and using
twice
and
in eq85 and simplifying gives
.
to
.