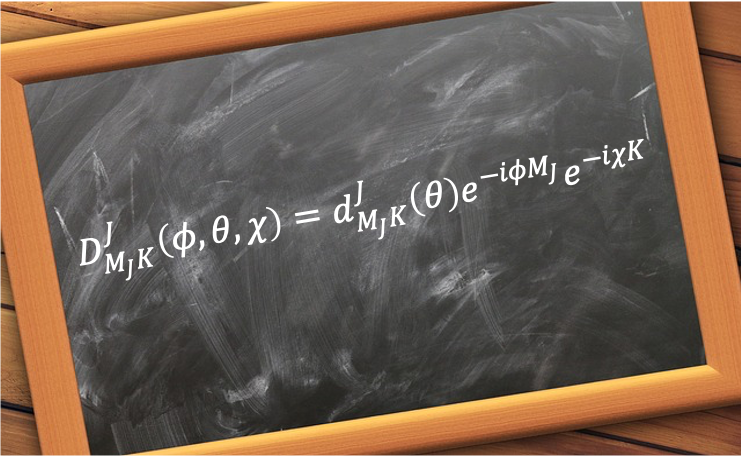The energy–time uncertainty relation states that the more precisely a system’s energy is defined, the longer it takes for the system to undergo a significant change.
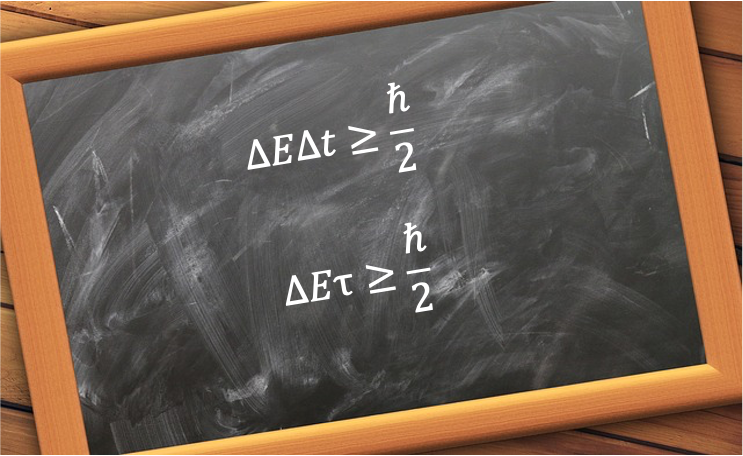
Mathematically, it is expressed as:
It can be derived from the general form of the uncertainty principle , where
and
are the observables corresponding to the Hermitian operators
and
, respectively. In this case, we let
be a system’s energy
, which is the observable associated with the Hamiltonian operator
. Any uncertainty in
therefore corresponds to a change in
.
However, in non-relativistic quantum mechanics, time is a parameter, not a Hermitian operator. Hence, we cannot replace
with a time operator
. Instead, we begin with the time evolution of the expectation value of an operator that does not explicitly depend on time:

Question
What is an operator that does not explicitly depend on time?
Answer
An operator that does not explicitly depend on time is one whose definition does not contain time as a variable. For example, the angular momentum operator depends only on spatial coordinates and not on time. However, the state
on which the operator acts may evolve with time according to the Schrödinger equation. Therefore, even though the operator itself is time-independent, its expectation value can still change over time as the state evolves.
Differentiating eq27b with respect to time using the product rule gives:
The time evolution of the state is governed by the time-dependent Schrödinger equation . Taking its Hermitian conjugate yields
. Substituting these two equations into eq27c results in:

Question
Explain the Hermitian conjugate forms of and
.
Answer
Linear operators acting on the Hilbert space vectors can be represented by square matrices. The Hermitian conjugate (or complex transpose) of two such matrices is given by (see property 13 of this article for proof). Therefore,
, where
. However,
is a scalar operator acting on a scalar parameter. If
, then
Comparing eq27d with the general uncertainty principle gives:
where .
Since , eq27e becomes the energy-time uncertainty relation. Here,
corresponds to the time scale for the system’s evolution, i.e. the time required for the expectation value of
to change by one standard deviation
.
An important example of this relation occurs in the excited state of a molecule, where corresponds to the lifetime
of the excited state, and
is the uncertainty in the transition energy between the excited and relaxed states. In other words, the shorter the lifetime of an unstable state, the larger the uncertainty in its transition energy. A large
means the emitted photon’s energy is not a single, sharp value, but a range of values, leading to a broadened line in the spectrum.
The broadening of spectral lines can also be caused by molecular interactions. For example, collisions between atoms or molecules lead to shortened excited-state lifetimes by inducing transitions via a non-radiative pathway. When two particles approach closely enough to interact, their potential energy varies according to the internuclear distance, producing a perturbation that couples their internal energy levels. If part of the internal energy of particle A, which is in an excited state, is transferred to particle B during the collision, particle A undergoes collisional de-excitation, and the excess energy is converted into additional kinetic energy of the colliding pair rather than being emitted as a photon. This process effectively reduces the lifetime of the excited state, leading to spectral broadening.
Finally, since , eq27a is sometimes written less precisely as:
or